Session Information
Date: Thursday, June 8, 2017
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: Characterize the levodopa pharmacokinetic profile derived from continuous subcutaneous (SC) administration of ND0612.
Background: Continuous levodopa/carbidopa (LD/CD) infusion is considered the optimal delivery route for patients with advanced PD. However, available delivery systems are limited by their requirement for invasive surgery. ND0612 is a proprietary liquid formulation of LD/CD administrated SC via a mini-pump delivering a range of LD doses from 270mg/day to 720mg/day.
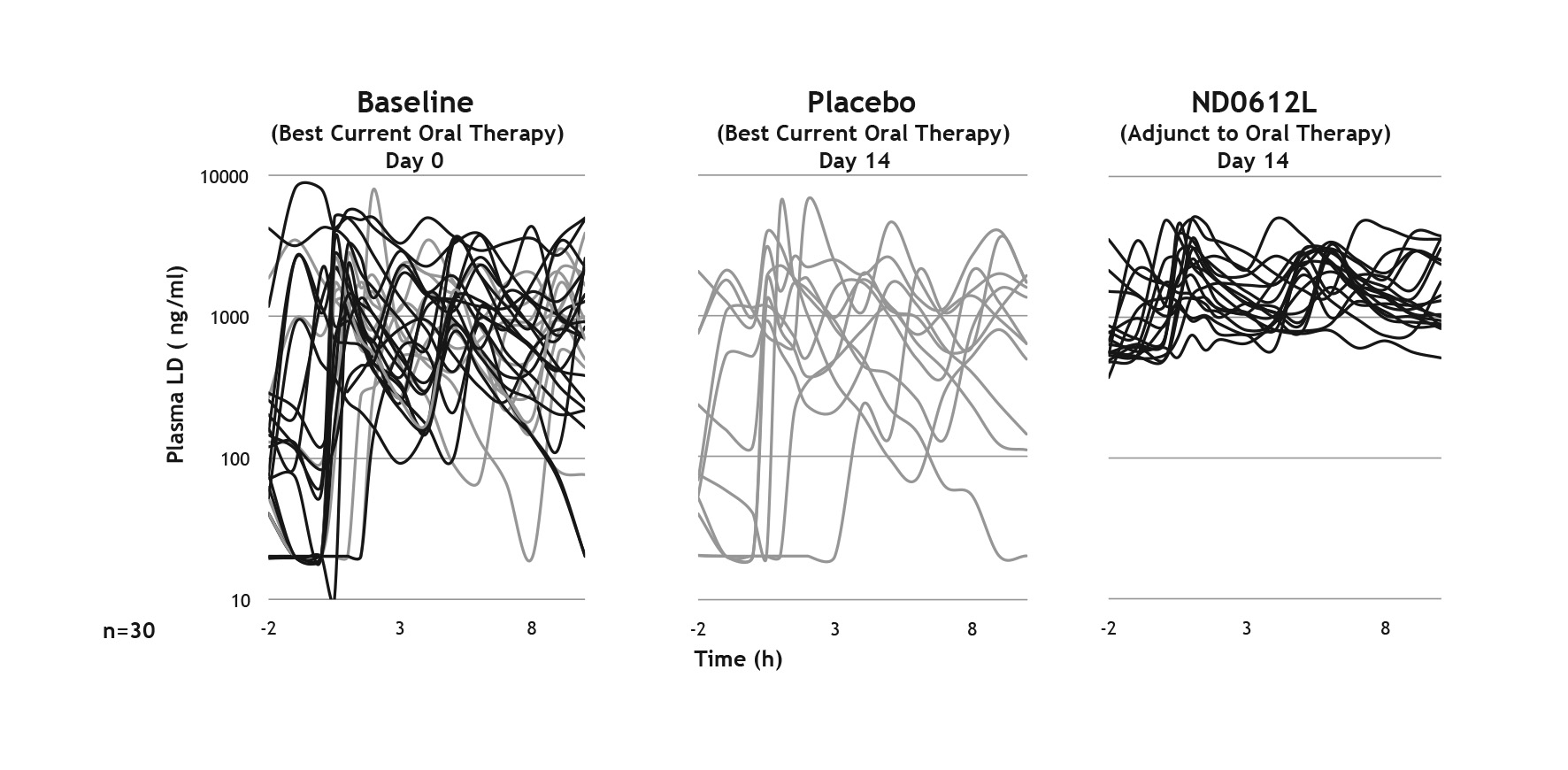
Methods: Two Phase II studies were performed. (1) Phase IIa study in which 16 PD patients were randomized to low or high dose ND0612 for 8-hours administration. (2) Double-blind, placebo-controlled study, in which 30 patients with PD received low dose ND0612 or placebo, as adjunct to their current optimized oral treatment for 2-weeks, followed by a 1-week open label extension of ND0612 either with or without oral entacapone.
Results: Study 1: fluctuations in LD plasma levels were markedly reduced (both doses) in comparison to baseline oral LD/CD. LD maximal plasma concentrations were dose proportionate with values of 487ng/mL and 1454ng/ml respectively for low and high ND0612 dose, respectively. Co-administration of entacapone BID increased LD plasma levels to 604ng/mL and 1844ng/mL in the low and high dose arms respectively. Study 2: adjunct treatment with low-dose ND0612 eliminated the LD troughs associated with oral LD/CD [Figure 1]. Furthermore, when oral LD was discontinued, peaks and troughs in LD plasma levels were abolished, and mean LD levels were maintained at 550ng/ml. Co-administered entacapone further increased LD levels to 800ng/ml. In addition, the trial met its futility analysis endpoint; adjunct ND0612 reduced mean OFF by 2.42h in clinic and 2.13h in pilot home diaries, (vs. 0.41h and 1.39h with optimized oral therapy and placebo).
Conclusions: Subcutaneous administration of ND0612 provides steady, therapeutic LD plasma concentrations that were associated with clinical improvement, and may provide an effective, non-invasive alternative for continuous LD delivery.
To cite this abstract in AMA style:
N. Giladi, Y. Caraco, T. Gurevich, R. Djaldetti, L. Adar, T. Rachmilewitz Minei, S. Oren. ND0612 (levodopa/carbidopa for subcutaneous infusion) achieves stable levodopa plasma levels when administered in low and high doses in patients with PD [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/nd0612-levodopacarbidopa-for-subcutaneous-infusion-achieves-stable-levodopa-plasma-levels-when-administered-in-low-and-high-doses-in-patients-with-pd/. Accessed January 27, 2026.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/nd0612-levodopacarbidopa-for-subcutaneous-infusion-achieves-stable-levodopa-plasma-levels-when-administered-in-low-and-high-doses-in-patients-with-pd/