Objective: To investigate the efficacy and safety of istradefylline for the treatment of postural abnormality in patients with Parkinson’s disease (PD).
Background: Postural abnormality in PD patients is often refractory to dopaminergic drugs, and currently, no established treatment approach exists. Istradefylline, an adenosine A2A receptor antagonist, is indicated as an adjunctive treatment to levodopa preparations in PD patients experiencing OFF time and is currently available in Japan and the US.
Method: In a multicenter, open-label, 24-week prospective study, PD patients with postural abnormality receiving levodopa treatment and experiencing wearing-off phenomenon were administered oral istradefylline for 6 months: 20 mg/day for 4 weeks followed by 40 mg/day. The primary endpoint was the mean change from baseline to week 24 in the 14-item Unified Dystonia Rating Scale (UDRS) total score. Mean changes in Movement Disorders Society Unified PD Rating Scale (MDS-UPDRS) part I and III, Freezing of Gait Questionnaire, Clinical Global Impression-Global Improvement, PD Questionnaire-8 scores, and Modified Hoehn and Yahr scale (ON/OFF state) were secondary endpoints.
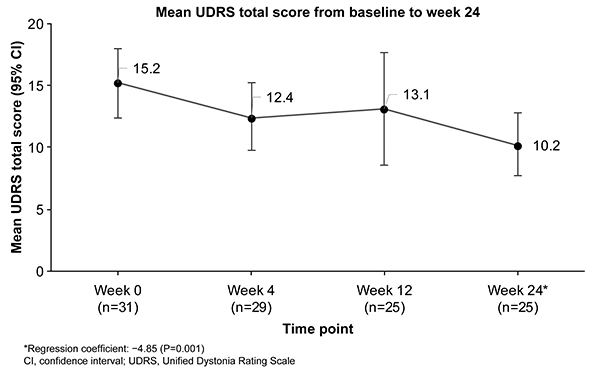
Results: Overall, 31 patients (female, 58.1%; mean [standard deviation] age, 73.3 [7.7] years) were enrolled. The mean (95% confidence interval [CI]) UDRS total score improved from baseline (15.2 [12.4, 18.0], n=25) over the study [Figure 1], with a significant mean (95% CI) improvement of 4.84 (1.97, 7.71; P=0.002 [by paired t-test]) at week 24. None of the severity scores for all 14 items of the UDRS worsened. The mean change (95% CI; regression coefficient) from baseline to week 24 in the MDS-UPDRS part III score was 7.84 (4.34, 11.34; −6.129; P<0.001 [by mixed effect model]). Other secondary endpoints did not change significantly. The most commonly reported adverse drug reactions (ADRs) were malaise, dyskinesia exacerbation, and visual hallucinations in 2 (6.5%) patients each. No serious ADRs were observed.
Conclusion: Topline results from this study demonstrated the efficacy and safety of istradefylline, a drug that acts by non-dopaminergic mechanism, in PD patients with postural abnormality. Istradefylline may be a potential treatment option for postural abnormality in PD patients treated with levodopa preparations and experiencing OFF time.
To cite this abstract in AMA style:
M. Takahashi, T. Shimokawa, J. Koh, T. Takeshima, H. Yamashita, Y. Kajimoto, A. Mori, H. Ito. Efficacy and safety of istradefylline in Parkinson’s disease patients with postural abnormality: Results from a multicenter, open-label study in Japan [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-istradefylline-in-parkinsons-disease-patients-with-postural-abnormality-results-from-a-multicenter-open-label-study-in-japan/. Accessed February 15, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-istradefylline-in-parkinsons-disease-patients-with-postural-abnormality-results-from-a-multicenter-open-label-study-in-japan/