Category: Parkinson’s Disease: Clinical Trials
Objective: To determine if nutritional ketosis supplemented by medium chain triglycerides (MCT) over the course of 2-3 weeks is acceptable and feasible in patients with idiopathic Parkinson’s disease (PD). We also explored the effect of nutritional ketosis on mobility as defined by Timed Up & Go (TUG) test.
Background: Converging evidence indicates mitochondrial insufficiency in the pathogenesis of Parkinson’s Disease. A growing body of literature supports ketogenic diet (KD) as a means to treat neurodegenerative disorders marked by mitochondrial depolarization/insufficiency. Studies of KD in PD show reduced non-motor symptom scores (UPDRS part 1) but there are limited data on feasibility of KD and no data on mobility scores.
Method: We conducted a six day randomized, double-blind, placebo-controlled MCT-supplemented KD (80% fat, 10-15% protein, <10% net carbohydrate (carb (total carb – fiber)) vs. standard diet (SD, 35% fat, 10-15% protein, 50-55% net carb) inpatient study, followed by 2 weeks of open label KD at home. The primary outcome was feasibility of the home KD at 3 weeks (retention>80%, diet favorability score >2/4, net carb intake <10% of energy based on unannounced dietary recalls). Secondary outcome was day 7 mean group difference in TUG scores.
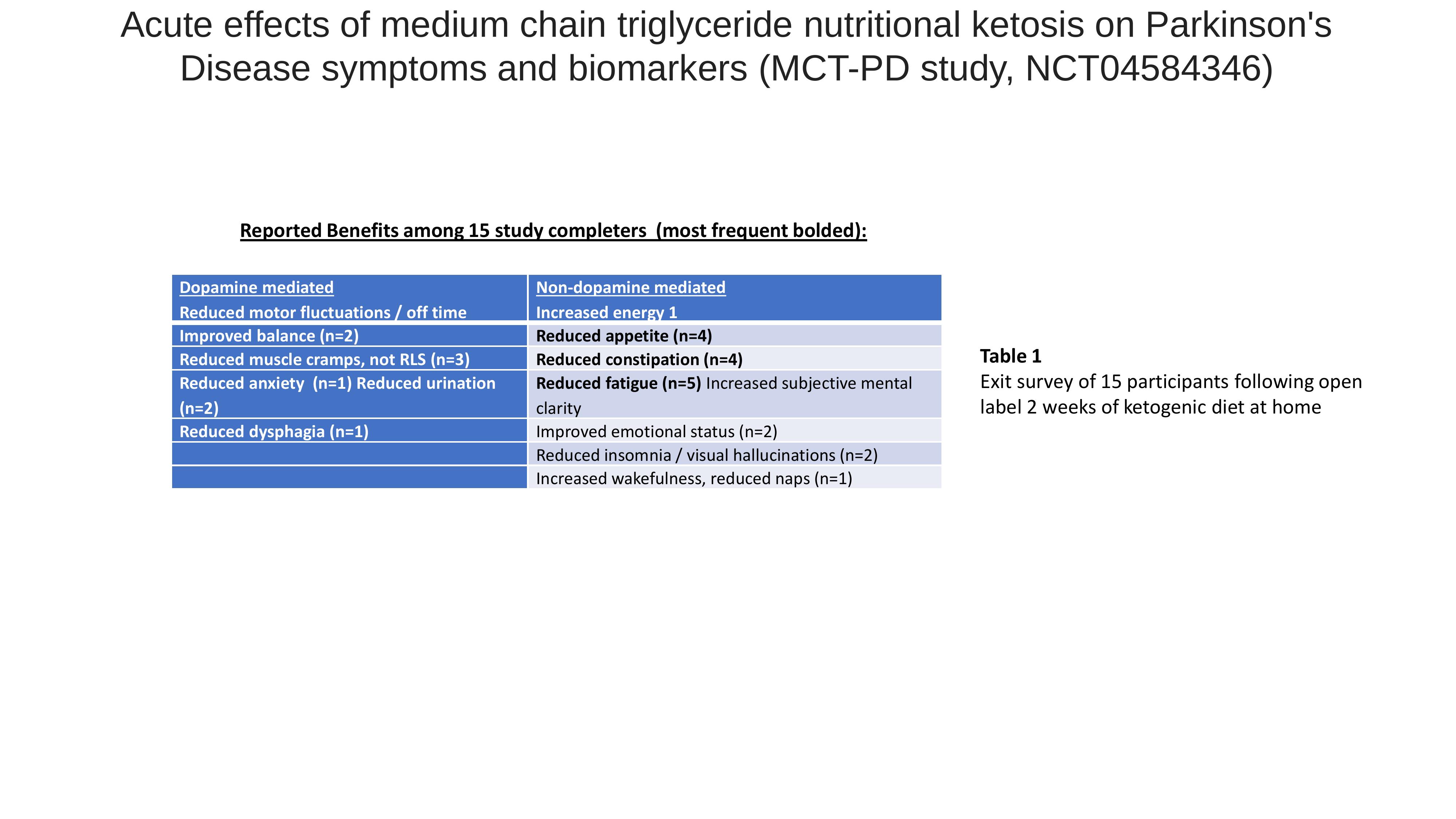
Results: 16 participants (mean age 67, BMI 27.6, Hoehn & Yahr stage 2-3) were randomized to ketogenic (KD n=7) or standard (SD, n=9) diets. 90% completed the study and there were no serious adverse events attributed to diet intervention. On day 7, mean TUG reduced in both groups (8.7 (1.1) to 8.2 (1.0)s KD, 10.2 (2.8) to 9.1 (2.3)s SD), and there was no significant KD-SD difference in baseline adjusted TUG score. At week 3, Net carb intake was 9.7%, mean Likert scale rating of 2.24/3 indicated somewhat to very likely to continue the KD in the future, and mean reduction in Non-Motor Symptoms Scale was seen in both groups (KD 46.1 to 24.4, SD 43.8 to 33.0).
Conclusion: MCT oil-supplemented KD was feasible and acceptable in PD patients, and provided critical data to power future studies. Further study with a longer follow-up interval and examination of nutritional ketosis on cognitive and other motor/non-motor outcomes is required to assess for potential disease modifying effects.
References: Van Itallie TB, Nonas C, Di Rocco A, Boyar K, Hyams K, Heymsfield SB. Treatment of Parkinson disease with diet-induced hyperketonemia: A feasibility study. Neurology. 2005;64:728–730.
Phillips MCL, Murtagh DKJ, Gilbertson LJ, Asztely FJS, Lynch CDP. Low-fat versus ketogenic diet in Parkinson’s disease: A pilot randomized controlled trial. Movement Disorders. 2018;33:1306–1314.
Krikorian R, Shidler MD, Summer SS, et al. Nutritional ketosis for mild cognitive impairment in Parkinson’s disease: A controlled pilot trial. Clinical Parkinsonism & Related Disorders. 2019;1:41–47.
To cite this abstract in AMA style:
A. Choi, M. Delgado, K. Chen, S. Chung, A. Courville, S. Turner, S. Yang, K. Airaghi, I. Dustin, P. Mcgurrin, M. Hallett, D. Ehrlich. A Pilot Feasibility Study of Medium Chain Triglyceride Nutritional Ketosis and Parkinson’s Disease [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/a-pilot-feasibility-study-of-medium-chain-triglyceride-nutritional-ketosis-and-parkinsons-disease/. Accessed January 31, 2026.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-pilot-feasibility-study-of-medium-chain-triglyceride-nutritional-ketosis-and-parkinsons-disease/