Category: Parkinson’s Disease: Clinical Trials
Objective: To report the efficacy and safety profiles of patients on sustained foslevodopa/foscarbidopa (LDP/CDP) continuous subcutaneous infusion (CSCI) mono- or polytherapy from three phase 3 clinical trials.
Background: As Parkinson’s disease (PD) progresses, patients often increase the number and classes of medications in an effort to control motor fluctuations. However, even those compliant with complex medical regimens can develop poor motor control. LDP/CDP (intended to replace oral levodopa [LD]/carbidopa [CD]) delivered 24 hours/day improves motor fluctuation and dyskinesia in patients with PD.
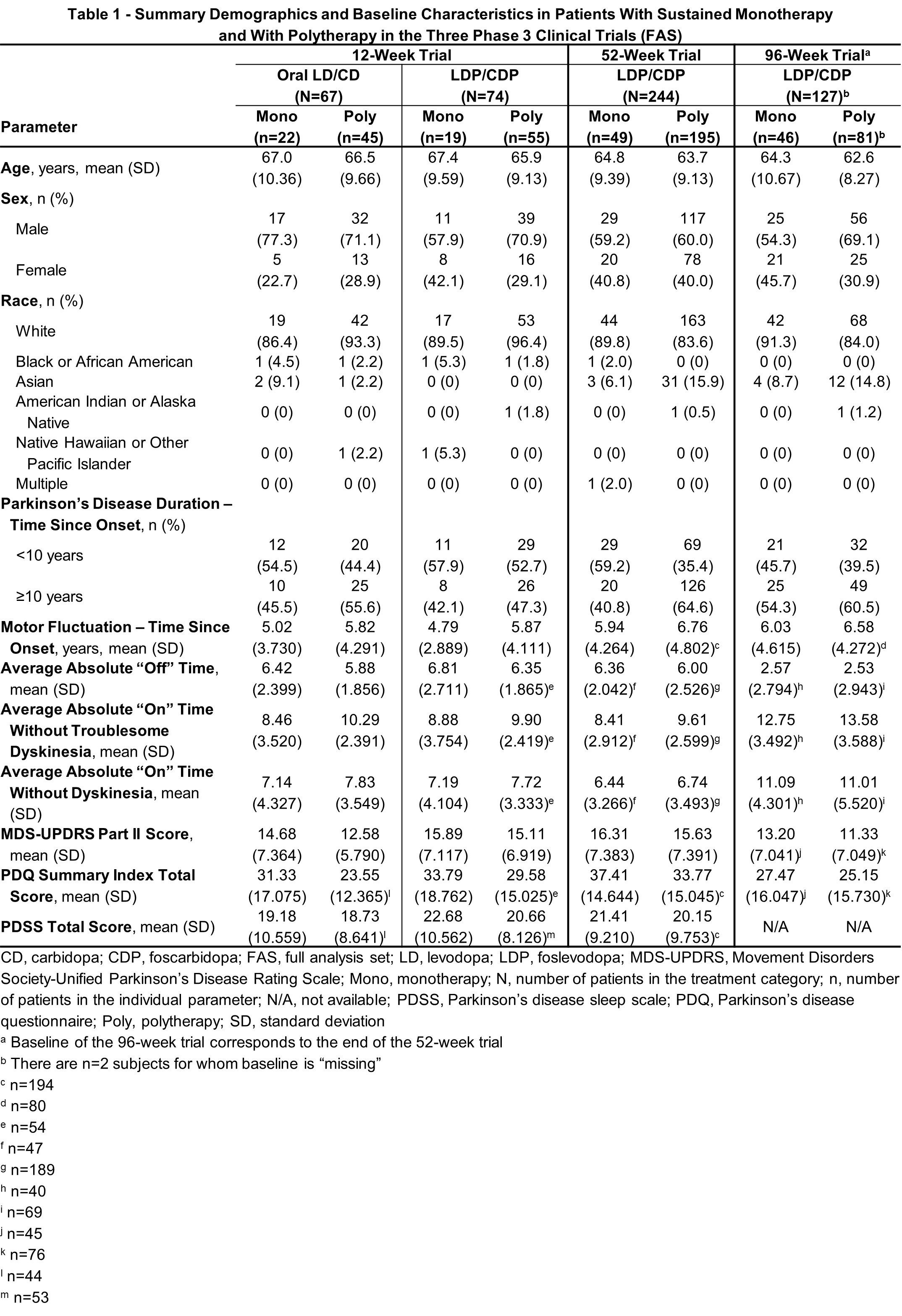
Method: This post-hoc analysis assessed patients with PD on sustained mono- or polytherapy in three LDP/CDP clinical trials, including: a randomized 12-week double-blind comparison of LDP/CDP CSCI versus oral LD/CD (NCT04380142), a single-arm 52-week open-label study of LDP/CDP CSCI (NCT03781167), and an open-label extension of the 52-week trial for up to 96-weeks (NCT04379050). Sustained monotherapy was defined as no concomitant PD medications used during LDP/CDP therapy (except in case of rescue in the 12-week trial).
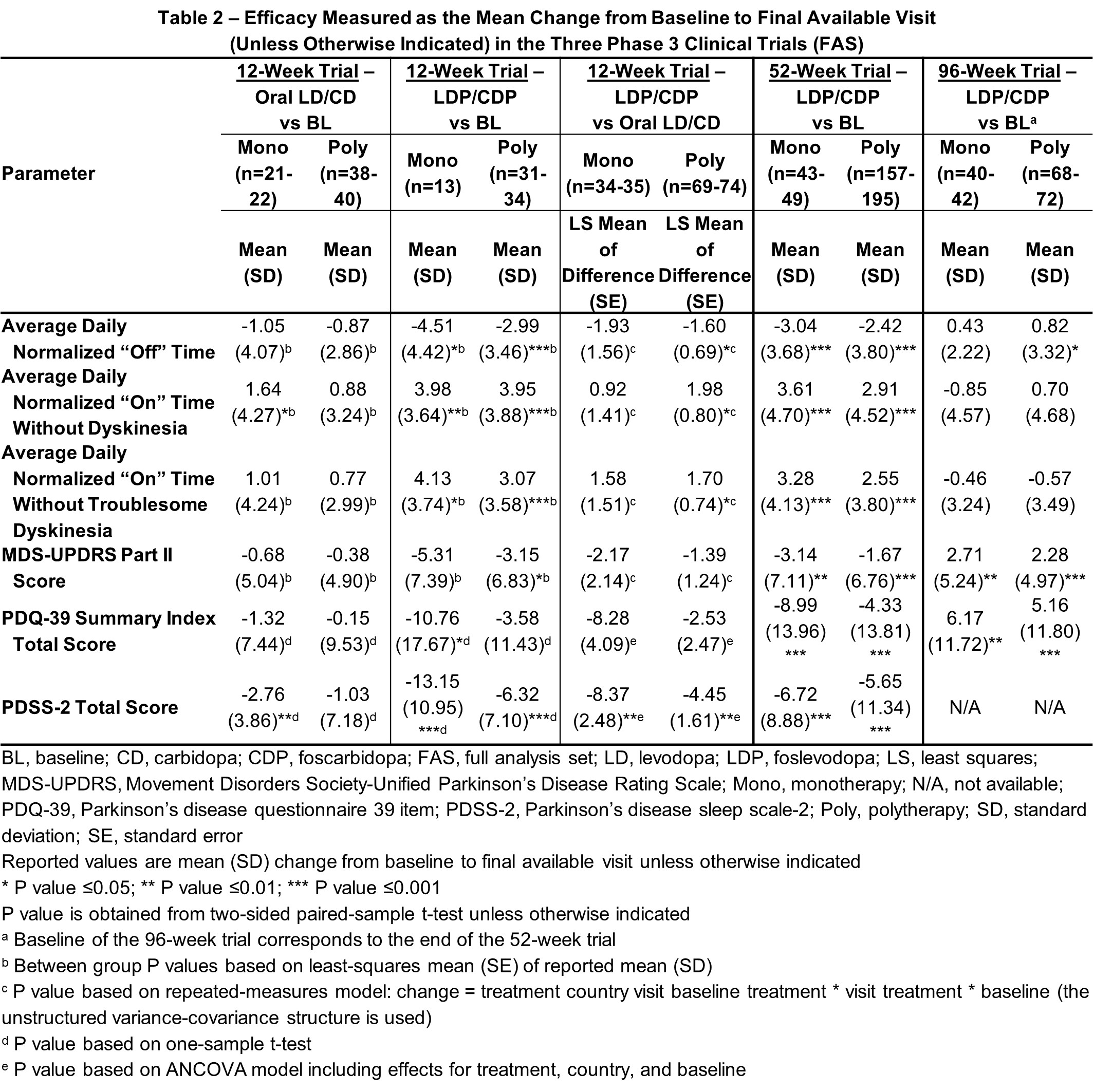
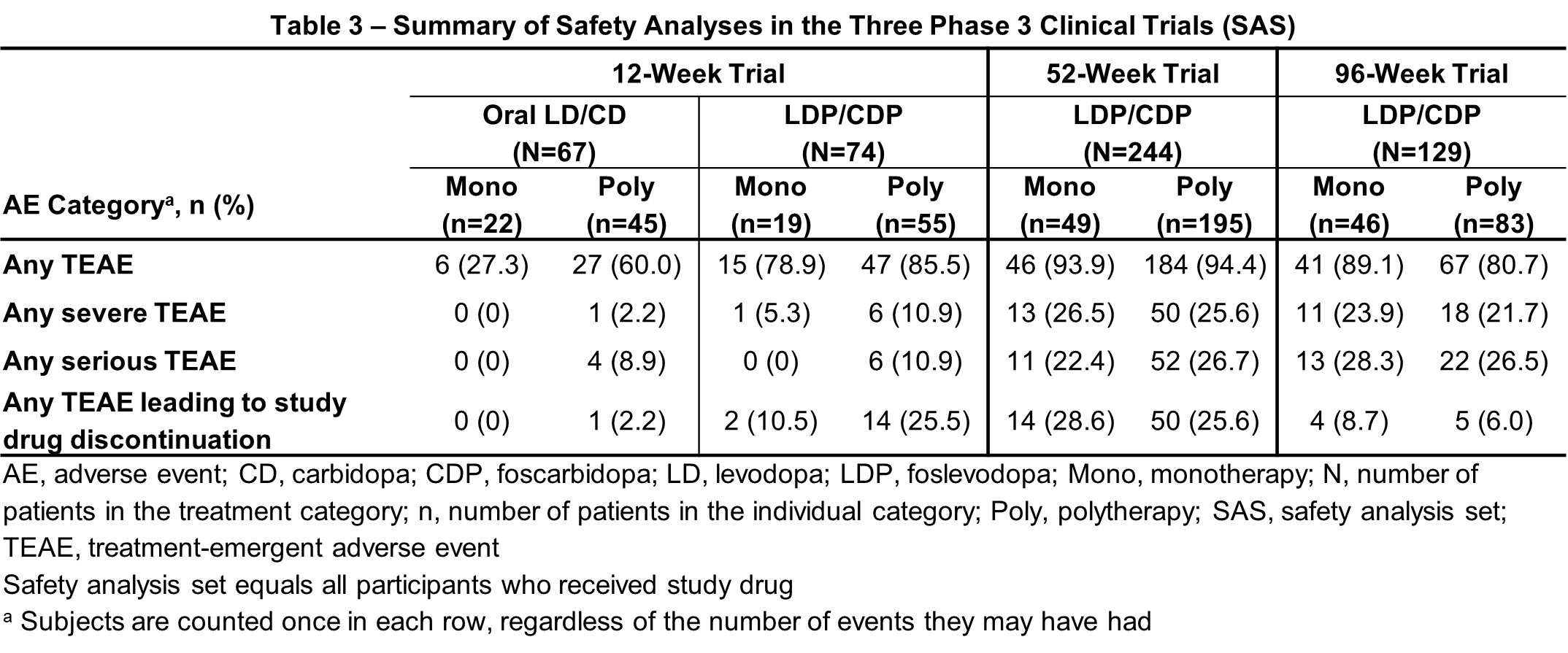
Results: Sustained LDP/CDP monotherapy was experienced by 25.7% of the 74 patients treated with LDP/CDP in the 12-week, 20.1% of the 244 patients in the 52-week, and 35.7% of the 129 patients in the 96-week trials [table1]. Consistent with the overall results, both mono- and polytherapy LDP/CDP groups demonstrated significant improvements vs baseline in “On” time without troublesome dyskinesia and “Off” time compared to oral [table2], with additional improvements vs baseline in key secondary endpoints also observed in the 12-week study. All outcomes in the 52-week trial showed significant improvement vs baseline with mono- and polytherapy. The 96-week extension trial with continued LDP/CDP therapy showed generally stable outcomes in both groups. Safety of mono- and polytherapy was overall similar in LDP/CDP-treated patients, with fewer events in the 12-week monotherapy group [table3].
Conclusion: In mono- and polytherapy-treated patients receiving LDP/CDP CSCI, overall comparable efficacy and safety profiles were demonstrated across the three phase 3 clinical trials. Thus, LDP/CDP monotherapy is achievable and can be considered a viable treatment option overall comparable to polytherapy.
To cite this abstract in AMA style:
J. Aldred, T. Henriksen, M. Bouchard, J. Martínez-Castrillo, M. Soileau, A. Spiegel, L. Bergmann, R. Gupta, P. Kukreja, D. Standaert. Efficacy and safety of foslevodopa/foscarbidopa continuous subcutaneous infusion administered as monotherapy in patients with Parkinson’s disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-foslevodopa-foscarbidopa-continuous-subcutaneous-infusion-administered-as-monotherapy-in-patients-with-parkinsons-disease/. Accessed February 3, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-foslevodopa-foscarbidopa-continuous-subcutaneous-infusion-administered-as-monotherapy-in-patients-with-parkinsons-disease/