Category: Parkinson’s Disease: Clinical Trials
Objective: To determine in Parkinson’s disease (PD) patients carrying GBA mutation the role of Ambroxol (ABX) on the progression of cognitive performance (primary aim), motor and non-motor symptoms and its effects at biochemical and brain imaging level (secondary aims)
Background: Heterozygous mutations in the GBA gene, encoding the lysosomal enzyme Glucocerebrosidase (GCase), are currently recognized as the most frequent genetic risk factor for PD.1 GBA-related PD (GBA-PD) patients have a higher risk of developing cognitive decline than non-carriers, resulting in poor quality of life and reduced survival.2 ABX is a chaperone that induces an increase in GCase levels and might interfere with PD progression3
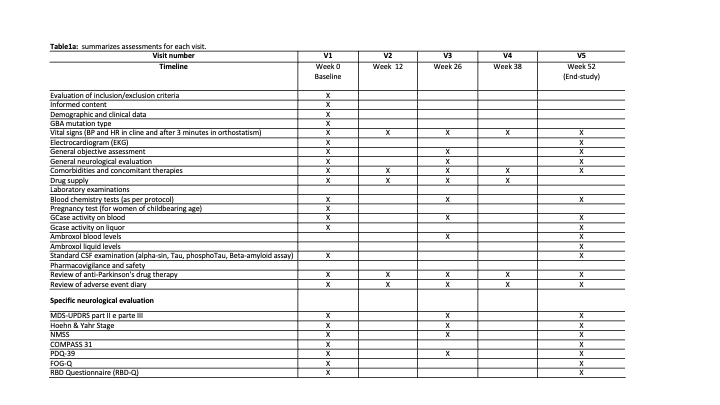
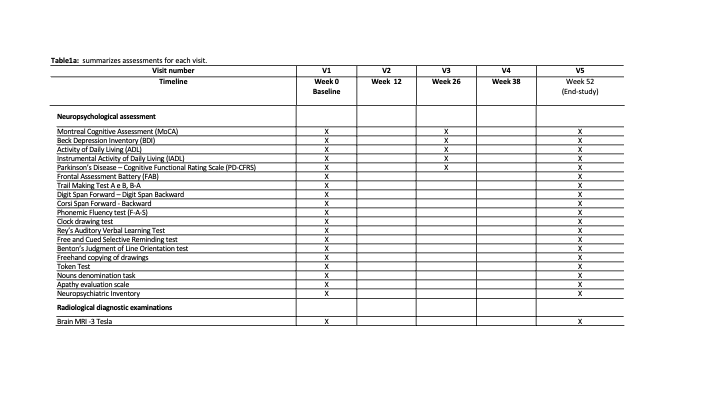
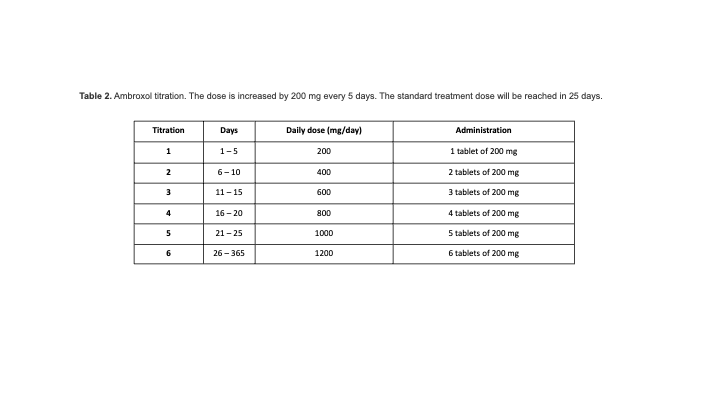
Method: This is a multicenter, double-blind, randomized, placebo-controlled phase II clinical trial, enrolling 60 GBA-PD. Patients will be randomized in 1:1 ratio to either ABX 1.2 g/day or placebo. Each participant will be assessed at baseline, weeks 12, 26, 38 and 52 and major outcome measure will be compared between baseline and after 52 weeks. ClinicalTrials.gov NCT05287503. EudraCT 2021-004565-13
Results: The Montreal Cognitive Assessment test will be used to measure the primary outcome. Secondary outcomes will evaluate by MDS-UPDRS parts II and III; H&Y; Composite Autonomic Symptom Score; Non-Motor Symptoms Scale; Freezing of gait questionnaire; REM sleep Behavior Disorder-questionnaire; PDQ-39 and a comprehensive battery of tests investigating all cognitive domains and activities of daily living. Neuroimaging features (cortical thickness, cortical and subcortical volumes, white matter microstructural damage, iron deposition and resting-state functional connectivity), pharmacokinetic and pharmacodynamic of ABX levels and GCase activity in plasma and cerebrospinal fluid will also be analyzed
Conclusion: To date, clinical trials failed to demonstrate efficacy of putative disease-modifying interventions on PD. Precision-medicine approaches targeting smaller cohorts with homogeneous well-defined molecular characteristics. ABX might be a potential disease-modifying therapy for GBA-PD. Considering the prominent impact of GBA mutations on the risk of incident dementia rather than motor disability, the primary objective is demonstrating a reduced progression of cognitive dysfunction over the 12-month period
References: 1. Lesage S, Anheim M, Condroyer C, Pollak P, Durif F, Dupuits C, Viallet F, Lohmann E, Corvol JC, Honoré A, Rivaud S, Vidailhet M, Dürr A, Brice A; French Parkinson’s Disease Genetics Study Group. Large-scale screening of the Gaucher’s disease-related glucocerebrosidase gene in Europeans with Parkinson’s disease. Hum Mol Genet. 2011 Jan 1;20(1):202-10. doi: 10.1093/hmg/ddq454
2. Zhang Y, Shu L, Zhou X, Pan H, Xu Q, Guo J, Tang B, Sun Q. A Meta-Analysis of GBA-Related Clinical Symptoms in Parkinson’s Disease. Parkinsons Dis. 2018 Sep 27;2018:3136415. doi: 10.1155/2018/3136415
3. McNeill A, Magalhaes J, Shen C, Chau KY, Hughes D, Mehta A, Foltynie T, Cooper JM, Abramov AY, Gegg M, Schapira AH. Ambroxol improves lysosomal biochemistry in glucocerebrosidase mutation-linked Parkinson disease cells. Brain. 2014 May;137(Pt 5):1481-95. doi: 10.1093/brain/awu020
To cite this abstract in AMA style:
F. Colucci, M. Avenali, R. de Micco, A. Bacila, S. Cerri, G. Cuconato, V. Franco, D. Franciotta, C. Ghezzi, M. Gastaldi, C. Galandra, G. Germani, P. Mitrotti, G. Ongari, I. Palmieri, M. Picascia, A. Pichiecchio, F. Esposito, M. Cirillo, F. Di Nardo, S. Aloisio, M. Siciliano, M. Fusar Poli, M. Stanziano, B. Garavaglia, F. Cazzaniga, C. Reale, I. Tramacere, S. Priori, P. Amami, S. Piacentini, MG. Bruzzone, M. Grisoli, F. Moda, R. Eleopra, A. Tessitore, EM. Valente, R. Cilia. Ambroxol as a disease-modifying treatment to reduce the risk of cognitive impairment in GBA-associated Parkinson’s disease.The AMBITIOUS Study Protocol [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/ambroxol-as-a-disease-modifying-treatment-to-reduce-the-risk-of-cognitive-impairment-in-gba-associated-parkinsons-disease-the-ambitious-study-protocol/. Accessed January 30, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/ambroxol-as-a-disease-modifying-treatment-to-reduce-the-risk-of-cognitive-impairment-in-gba-associated-parkinsons-disease-the-ambitious-study-protocol/