Objective: Describe the design of a phase 2 study (NCT05642442) of a once-daily, extended-release formulation of suvecaltamide to treat moderate to severe Parkinson’s disease tremor (PDT).
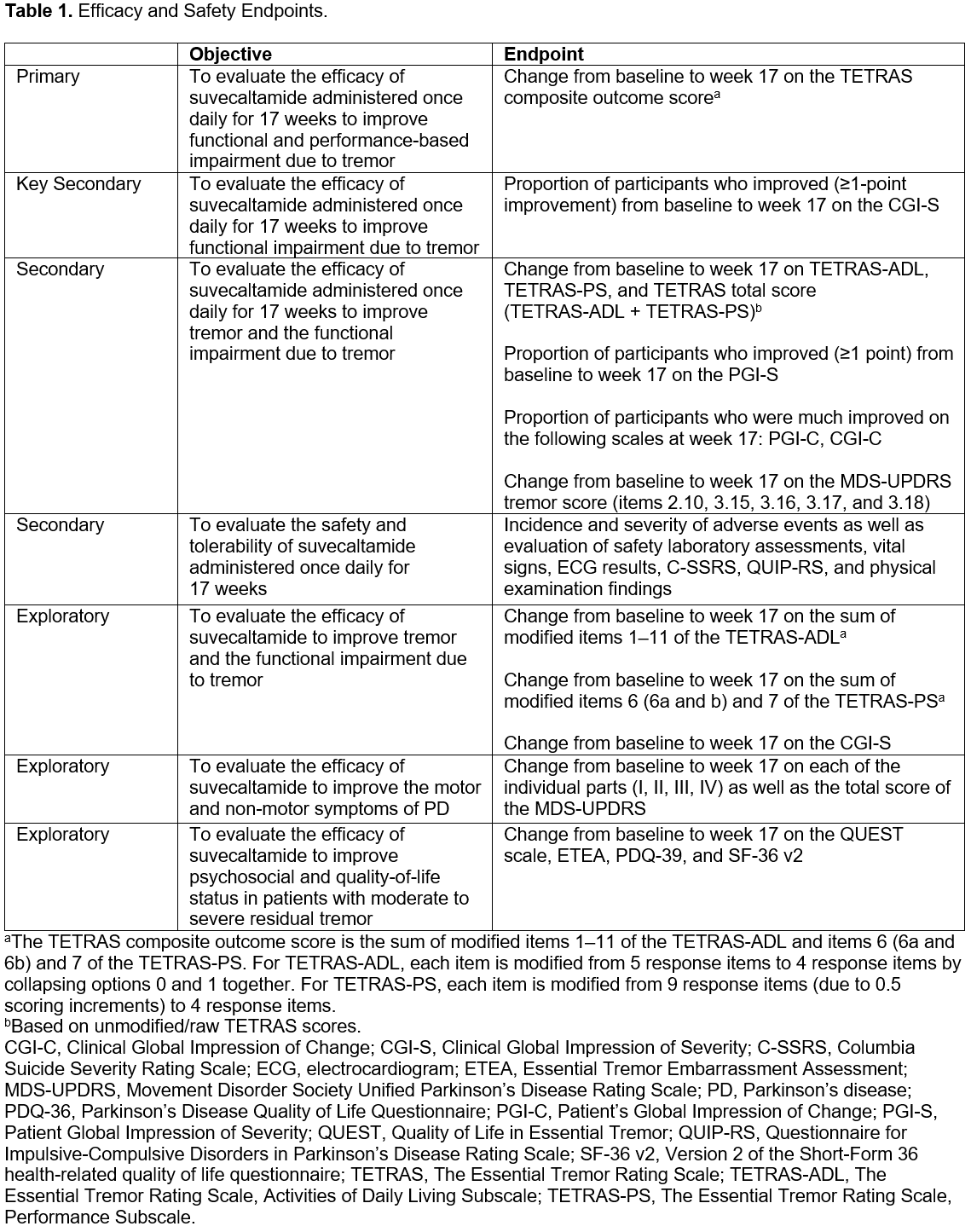
Background: Suvecaltamide, a T-type calcium channel modulator, is a nondopaminergic medication in development for treatment of PDT.[1] Tremor is one of the most troublesome features of PD and is often poorly controlled by standard dopaminergic medications.[2][3] Clinical assessments primarily focus on tremor amplitude, but the functional impact of tremor may represent a more clinically meaningful measure. This phase 2 study will evaluate the efficacy of suvecaltamide on the functional impact of PDT using a TETRAS composite outcome score (TETRAS-ADL items 1–11, and -PS items 6 & 7), as well as several secondary and exploratory scales.
Method: This double-blind, placebo-controlled, flexible-dosing, parallel-group, multicenter study will enroll participants with PD (aged 40−80 years) who have inadequately controlled tremor despite optimized treatment with PD medications. Exclusion criteria include unpredictable on/off periods and tremor only during “off” periods (for those who experience motor fluctuations). Participants will be randomized 1:1 to placebo or suvecaltamide, stratified by baseline TETRAS composite score (>17 or ≤17). In a 5-week titration period, participants will receive suvecaltamide 5 mg/day for 1−2 weeks, increasing to 10 mg/day for 1 week, and then increasing by 10 mg/day once per week up to 30 mg/day to optimize efficacy and tolerability. A 12-week maintenance period follows. The primary endpoint is change from baseline at week 17 on a TETRAS composite score [table 1]. Additional endpoints will assess PD symptoms, clinician and participant global impressions, embarrassment and quality of life due to tremor, and safety and tolerability.
Results: Target enrollment is 160 participants (128 evaluable) with 80% power to detect a difference of 3.0 points for change from baseline to week 17 on the primary endpoint between suvecaltamide and placebo (common σ=6.0, 2-sided α=0.05). Enrollment is ongoing.
Conclusion: The phase 2 study will serve as proof-of-concept for the efficacy of suvecaltamide to treat the functional impact of tremor in Parkinson’s disease and represents the first large clinical study to inform the use of TETRAS to assess tremor in this patient population.
References: 1. Papapetropoulos S, et al. Mov Disord. 2021;36:1944-49.
2. Pasquini J, et al. Brain. 2018;141:811-21.
3. Gupta DK, et al. Tremor Other Hyperkinet Mov (N Y). 2020;10:58.
To cite this abstract in AMA style:
R. Hauser, C. Adler, G. Deuschl, M. Baladi, P. Chandler, T. Skarpass, K. Xu, P. Lewitt. Design of a Phase 2 Study of Suvecaltamide in Moderate to Severe Parkinson’s Disease Tremor [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/design-of-a-phase-2-study-of-suvecaltamide-in-moderate-to-severe-parkinsons-disease-tremor/. Accessed February 27, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/design-of-a-phase-2-study-of-suvecaltamide-in-moderate-to-severe-parkinsons-disease-tremor/