Category: Rare Genetic and Metabolic Diseases
Objective: Minimum clinically important differences (MCID) on the Unified Wilson Disease Rating Scale (UWDRS) Part III were estimated using data from a phase III trial of tiomolibdate choline (TMC; INN: tiomolibdic acid; ALXN1840) vs standard of care (SoC) in patients (pts) with Wilson disease (WD).
Background: FoCus is an open-label randomized trial (NCT03403205) of 214 pts with WD aged ≥12 years. Pts were stratified by duration of prior SoC (cohort 1: >28 days; cohort 2: 0–28 days) and randomized 2:1 to either TMC (15mg QOD–60mg QD) or SoC (penicillamine, trientine, and/or zinc) for 48 weeks (W). The UWDRS Part III is a clinician-reported scale designed to evaluate neurologic manifestations of WD; higher score indicates greater severity. MCID is the degree of change that may be considered to represent a clinically meaningful difference on an assessment. There are no published MCID estimates for this scale, but an increase of 4 points1,2 or 20%3 has been assumed to represent meaningful worsening. This is a pilot study on MCID thresholds for the UWDRS III.
Method: Distribution-based MCIDs using standard deviation (1/3*SD) and the standard error of measurement (SEM) were calculated based on the variation in UWDRS III change scores that were observed. MCIDs were calculated for the overall pt population and for those with incomplete and/or intolerant response (IIR) to prior treatment. The IIR group was prespecified as pts with baseline UWDRS Part II or III total score >0 or history of treatment discontinuation due to intolerance or an adverse event leading to drug withdrawal between screening and first dose of study treatment.
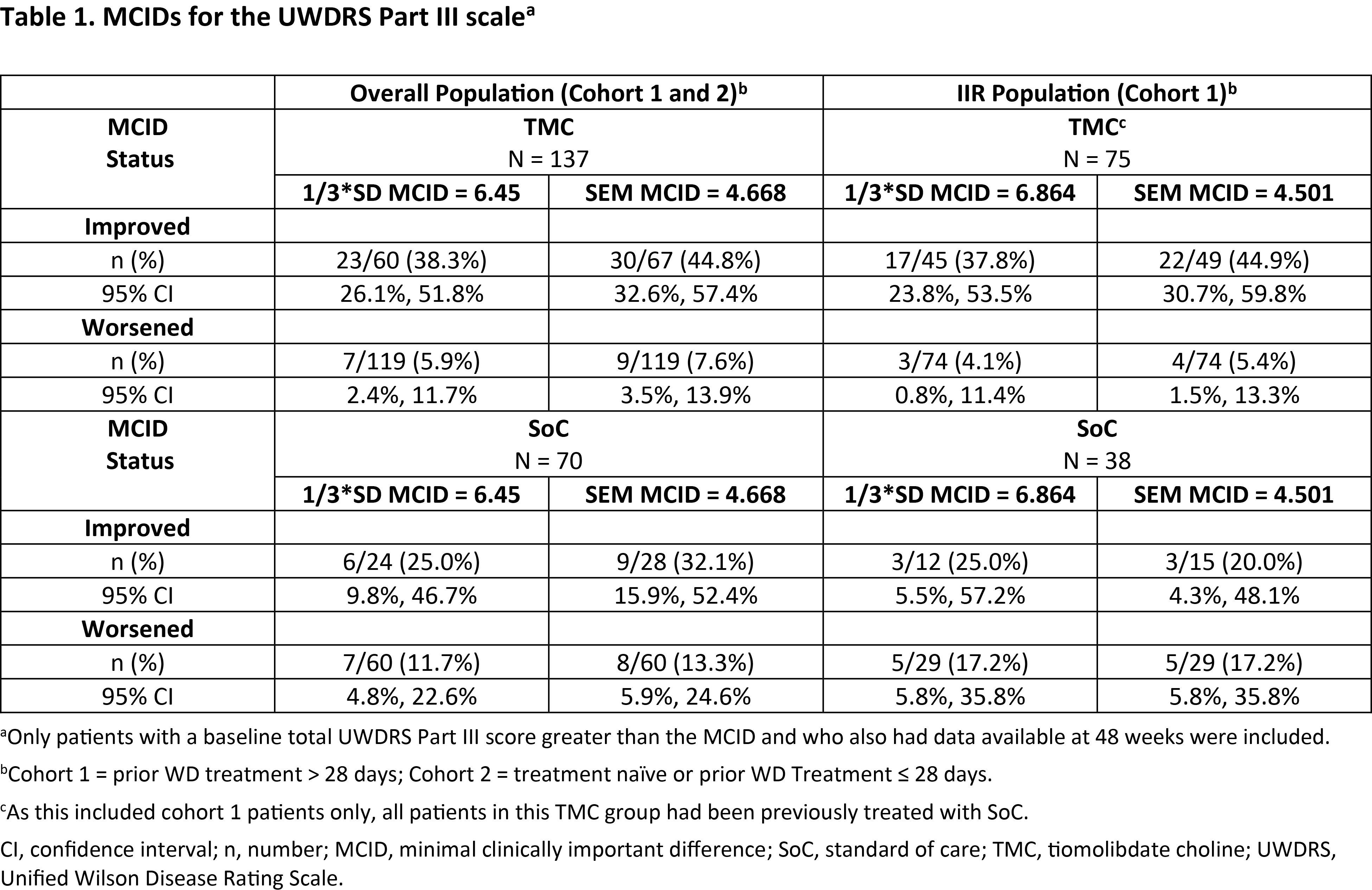
Results: 1/3*SD and SEM MCIDs were 6.45 and 4.668 (overall population) and 6.864 and 4.501 (IIR group), respectively (Table 1). For the 1/3*SD MCIDs, in the overall population, 38.3% of pts treated with TMC and 25.0% of pts treated with SoC had meaningful improvement and 5.9% (TMC) and 11.7% (SoC) had meaningful worsening after 48W. Results were similar in the IIR group (improvement: 37.8% vs 25.0%; worsening: 4.1% vs 17.2%). These results were analogous to those derived using SEM. The treatment groups had similar results.
Conclusion: Estimated UWDRS III distributional MCIDs obtained from the study were consistent with clinical judgments in the literature.1-3 The MCID range was determined to be 4.5 to 6.9 points in this WD population.
References: 1 Czlonkowska et al. Eur J of Neur 2014, 21:599-606.
2 Litwin et al. J Neurol Sci 2015, 355:162-167.
3 Poujois et al. Neurology 2020, 94:1-e14.
To cite this abstract in AMA style:
T. Litwin, A. Czlonkowska, P. Hedera, J. Bronstein, M. Lorincz, M. Møller, G. Wegmann, G. Carron, A. Messali, A. Poujois. Minimum clinically important difference (MCID) on the Unified Wilson Disease Rating Scale (UWDRS) Part III: results from the Phase 3 FoCus Trial [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/minimum-clinically-important-difference-mcid-on-the-unified-wilson-disease-rating-scale-uwdrs-part-iii-results-from-the-phase-3-focus-trial/. Accessed February 11, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/minimum-clinically-important-difference-mcid-on-the-unified-wilson-disease-rating-scale-uwdrs-part-iii-results-from-the-phase-3-focus-trial/