Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate safety and efficacy of foslevodopa/foscarbidopa (LDP/CDP) for patients with Parkinson’s disease (PD) in a 52-week, phase 3 study.
Background: As PD progresses, patients often require advanced treatments to manage their symptoms. LDP/CDP is a soluble formulation of levodopa/carbidopa (LD/CD) prodrugs delivered as a continuous (24-hour/day) subcutaneous infusion (CSCI) that can be customized to deliver a range of 600−4250 mg LD equivalents/day. A phase 3, double-blind, randomized controlled trial demonstrated LDP/CDP efficacy and safety vs oral immediate release LD/CD [1].
Method: This 52-week, phase 3, single-arm, open-label study (NCT03781167) enrolled patients with PD whose symptoms were not adequately controlled on their current therapies (≥2.5 hours “Off” time/day). LDP/CDP and permitted concomitant medications were titrated during a 4-week optimization phase. Further adjustments to LDP/CDP (but not concomitant PD medication, unless medically necessary) could be made during the 48 week maintenance phase. Additional lower and/or higher infusion rates could be pre-programmed by physicians to accommodate patients’ changing needs through the day. The primary endpoint was safety/tolerability. Secondary endpoints included “Off”/“On” times (PD diary), and Parkinson’s Disease Sleep Scale-2 (PDSS-2) scores.
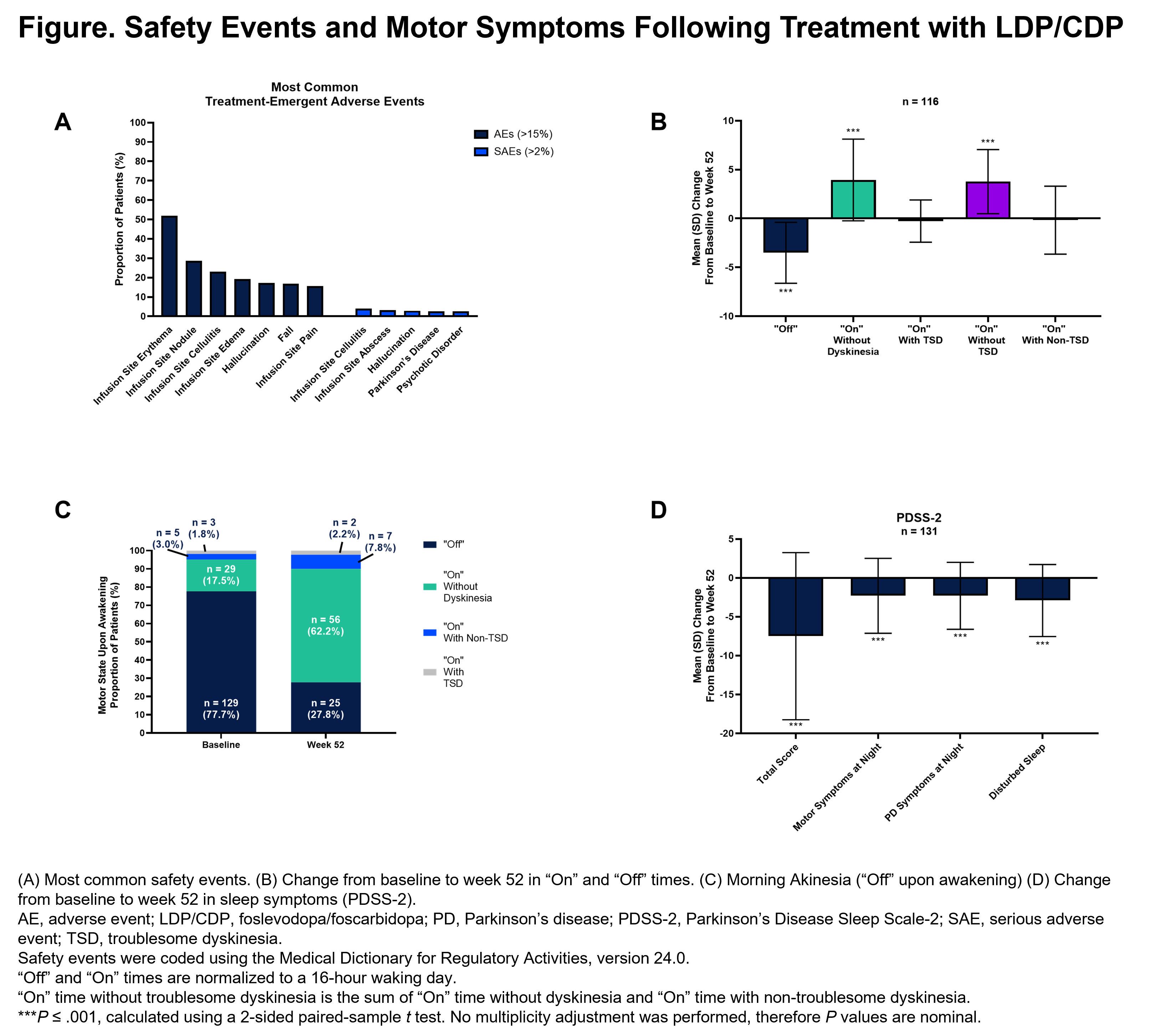
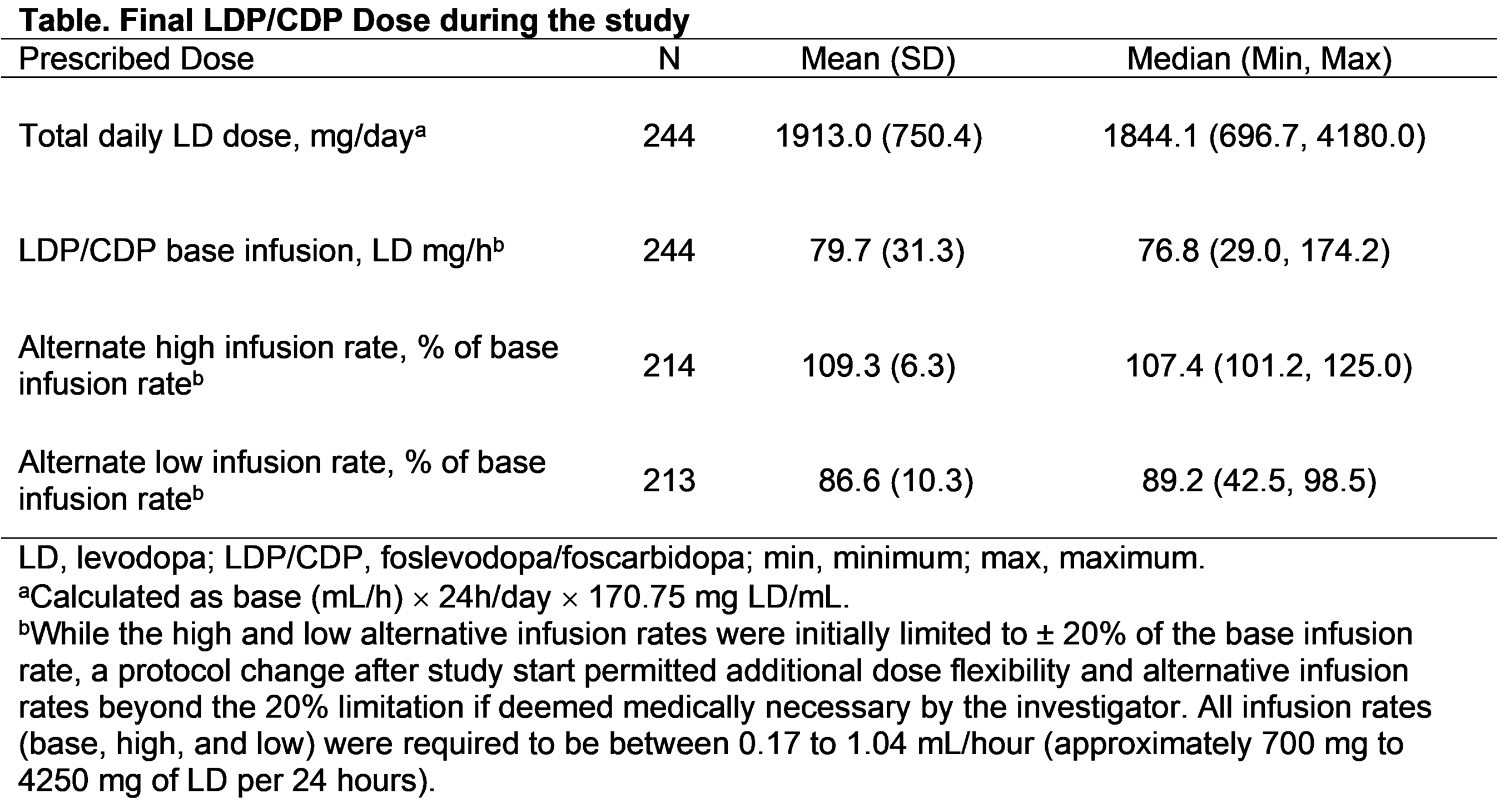
Results: Of 244 enrolled patients (male, [59.8%]; White, [84.8%]; mean (SD) age, 63.9 [9.2] years; daily “Off” time, 5.9 [2.2] hours), 137 completed and 107 prematurely discontinued LDP/CDP. The most common adverse events (AEs) were infusion site events (reaction, 82.0%; infection, 35.2%); the majority of AEs were non-serious and mild/moderate in severity [figure]. ”Off” time improved from the first post-baseline visit to week 52 (mean [SD] change: −3.5 [3.1] hours). Morning akinesia (patients reporting “Off” upon awakening) decreased from baseline (77.7%) to week 52 (27.8%). Sleep (PDSS-2) improved from baseline to week 52. LDP/CDP allowed for dose customization, as shown by the minimum and maximum alternate infusion rates at the end of the study (high: 101.2% to 125.0% of the base infusion rate; low: 42.5% to 98.5%) [table]. Of the 137 patients who completed the study, 129 (94.2%) enrolled in the open-label extension.
Conclusion: LDP/CDP 24-hour CSCI had a favorable benefit/risk profile and may provide an efficacious, individualized, non-surgical treatment option for patients with advanced PD.
References: 1. Soileau MJ, et al. Lancet Neurol. 2022;21:1099–1109.
To cite this abstract in AMA style:
J. Aldred, A. Amelin, A. Antonini, B. Bergmans, F. Bergquist, M. Bouchard, K. Budur, C. Carroll, K. Chaudhuri, S. Criswell, E. Danielsen, E. Freire Alvarez, F. Gandor, J. Jia, T. Kimber, H. Mochizuki, W. Robieson, A. Spiegel, D. Standaert, S. Talapala, M. Facheris, V. Fung. Continuous subcutaneous foslevodopa/foscarbidopa: final results from a phase 3, open-label study [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/continuous-subcutaneous-foslevodopa-foscarbidopa-final-results-from-a-phase-3-open-label-study/. Accessed February 16, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/continuous-subcutaneous-foslevodopa-foscarbidopa-final-results-from-a-phase-3-open-label-study/