Category: Parkinson’s Disease: Clinical Trials
Objective: To characterize infusion site events (ISEs) in patients with advanced Parkinson’s disease (aPD) treated with foslevodopa/foscarbidopa (LDp/CDp).
Background: ISEs, including local reactions and infusion site infections, are common during continuous subcutaneous infusion (CSCI) therapy [1]. LDp/CDp is a formulation of levodopa/carbidopa prodrugs administered as a 24‑hour/day CSCI. The systemic safety profile of LDp/CDp was consistent with that of oral levodopa/carbidopa [2,3]. As with other CSCI therapies, ISEs were reported with LDp/CDp [2,3].
Method: This post hoc analysis was conducted in patients receiving LDp/CDp in a 12-week, phase 3, randomized study (NCT04380142). Patients were ≥30 years old with idiopathic, levodopa-responsive aPD that was inadequately controlled by current therapy (≥2.5 “Off” hours/day). ISEs were recorded by investigators (who could report multiple different ISEs for any given event), including median time to onset/resolution and severity. Clinical management of presumed infusion site infections were summarized for the 3 most commonly reported preferred terms (infusion site infection, infusion site cellulitis, and infusion site abscess). Infusion site cultures were infrequently performed; therefore, infections were presumed in most cases.
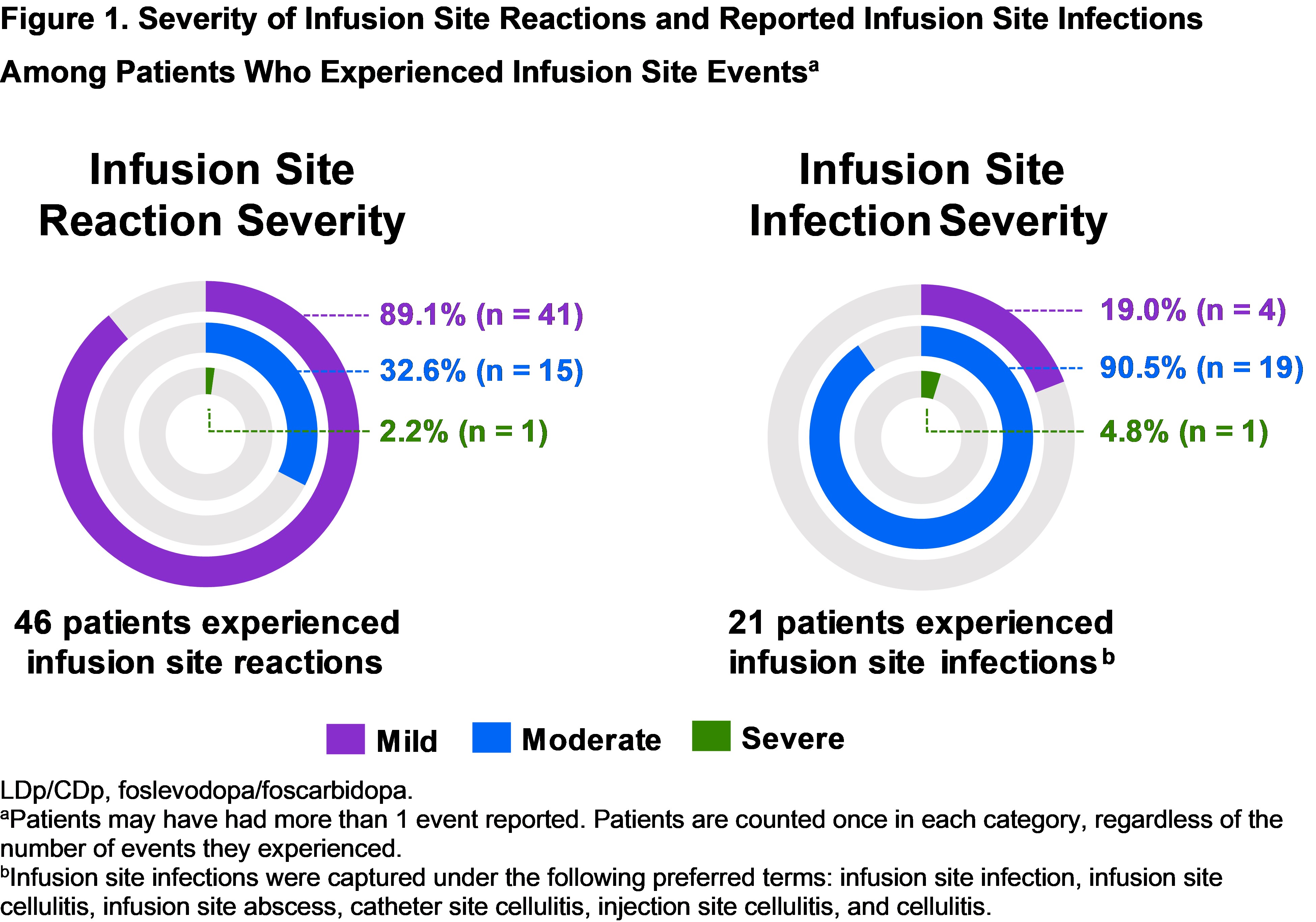
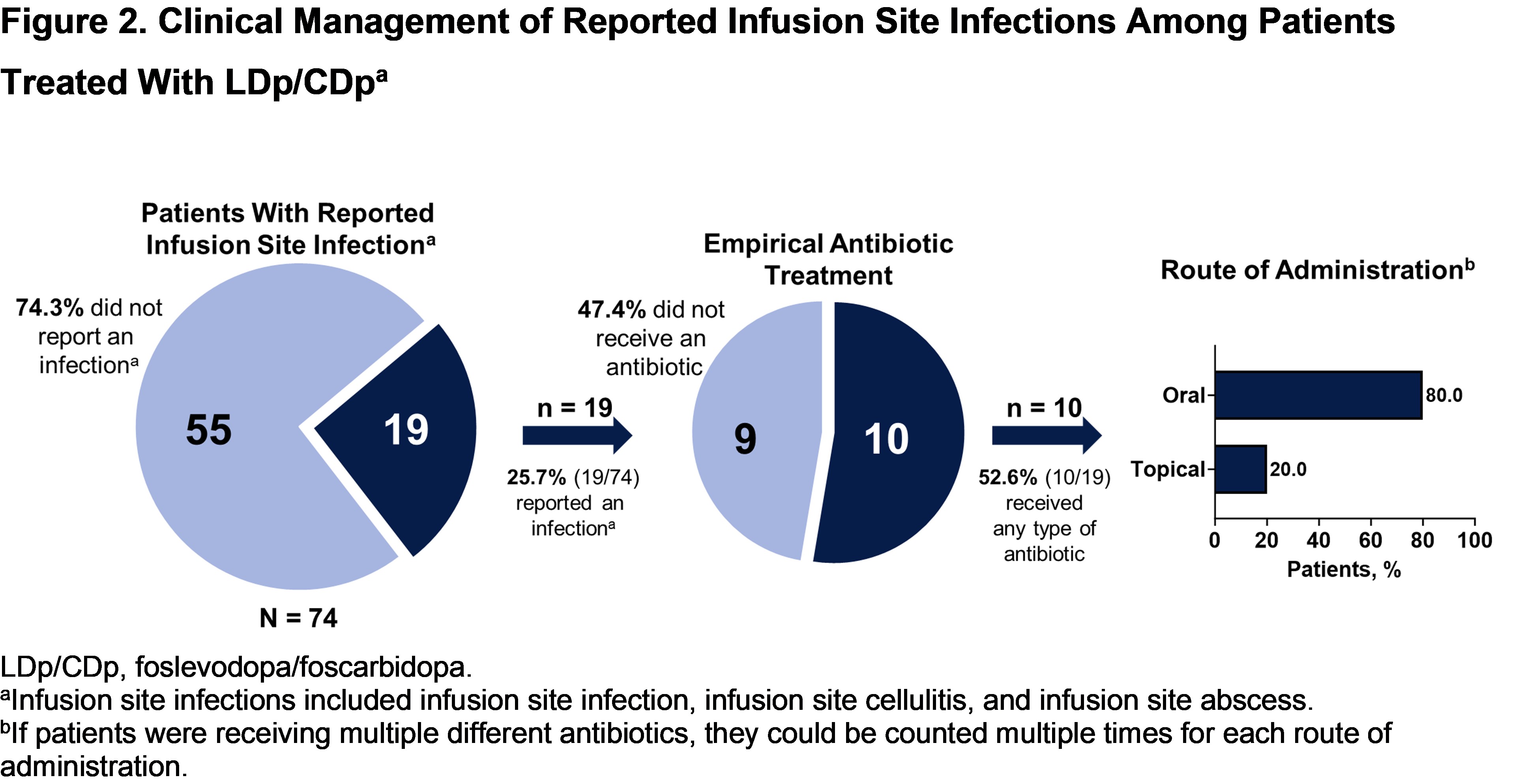
Results: Of 74 patients receiving LDp/CDp, infusion site reactions were reported in 46 (62.2%) and presumed infusion site infections in 21 (28.4%). Of these, most experienced infusion site reactions/infections that were mild or moderate in severity (45/46 [97.8%] and 20/21 [95.2%], respectively) [figure1]; 2 patients experienced serious infusion site infections. The median time to onset of infusion site reactions/infections was 5 and 32 days, respectively; median time to resolution was 7 and 18 days, respectively. Most patients who experienced ISEs continued LDp/CDp. Roughly half of presumed infections were empirically treated with oral antibiotics and resolved with or without treatment [figure2].
Conclusion: Presumed infusion site infections represented a small subset of ISEs; most were non-serious, mild to moderate in severity, did not result in discontinuation, and resolved with or without antibiotics. Better outcomes may result when patients/care partners use aseptic techniques, rotate infusion sites more often than every third day, use a new infusion set upon changes in the skin, and monitor for early infection signs.
Figure 1
Figure 2
References: 1. Jick S, et al. Curr Med Red Opin. 2021;37:1563–1571.
2. Soileau MJ, et al. Lancet Neurol. 2022;21:1099–1109.
3. Aldred J, et al. Neurol Ther. 2023;12:1937–1958.
To cite this abstract in AMA style:
S. Isaacson, R. Kirsner, O. Vaou, R. Pahwa, R. Hauser, L. Bergmann, R. Gupta, M. Shah, S. Varughese, T. Kimber. Evaluation of Infusion Site Adverse Events With Foslevodopa/Foscarbidopa in a 12-Week, Randomized Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/evaluation-of-infusion-site-adverse-events-with-foslevodopa-foscarbidopa-in-a-12-week-randomized-study/. Accessed February 3, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/evaluation-of-infusion-site-adverse-events-with-foslevodopa-foscarbidopa-in-a-12-week-randomized-study/