Category: Tremor
Objective: Evaluate the efficacy and safety of JZP385 (formerly CX-8998), a highly selective T-type calcium channel modulator, for symptomatic control of moderate to severe essential tremor (ET).
Background: ET is a progressive neurological disorder that can profoundly impact activities of daily living (ADL) [1, 2]. ET has no cure, and up to half of patients are unresponsive to current symptomatic treatments [3]. A previous phase 2a, 4-week proof-of-concept study of an immediate-release formulation of JZP385 administered twice-daily supported its continued clinical investigation in ET. The current phase 2b study explores further the efficacy/safety of 3 doses of a delayed-release once-daily (QD) formulation of JZP385.
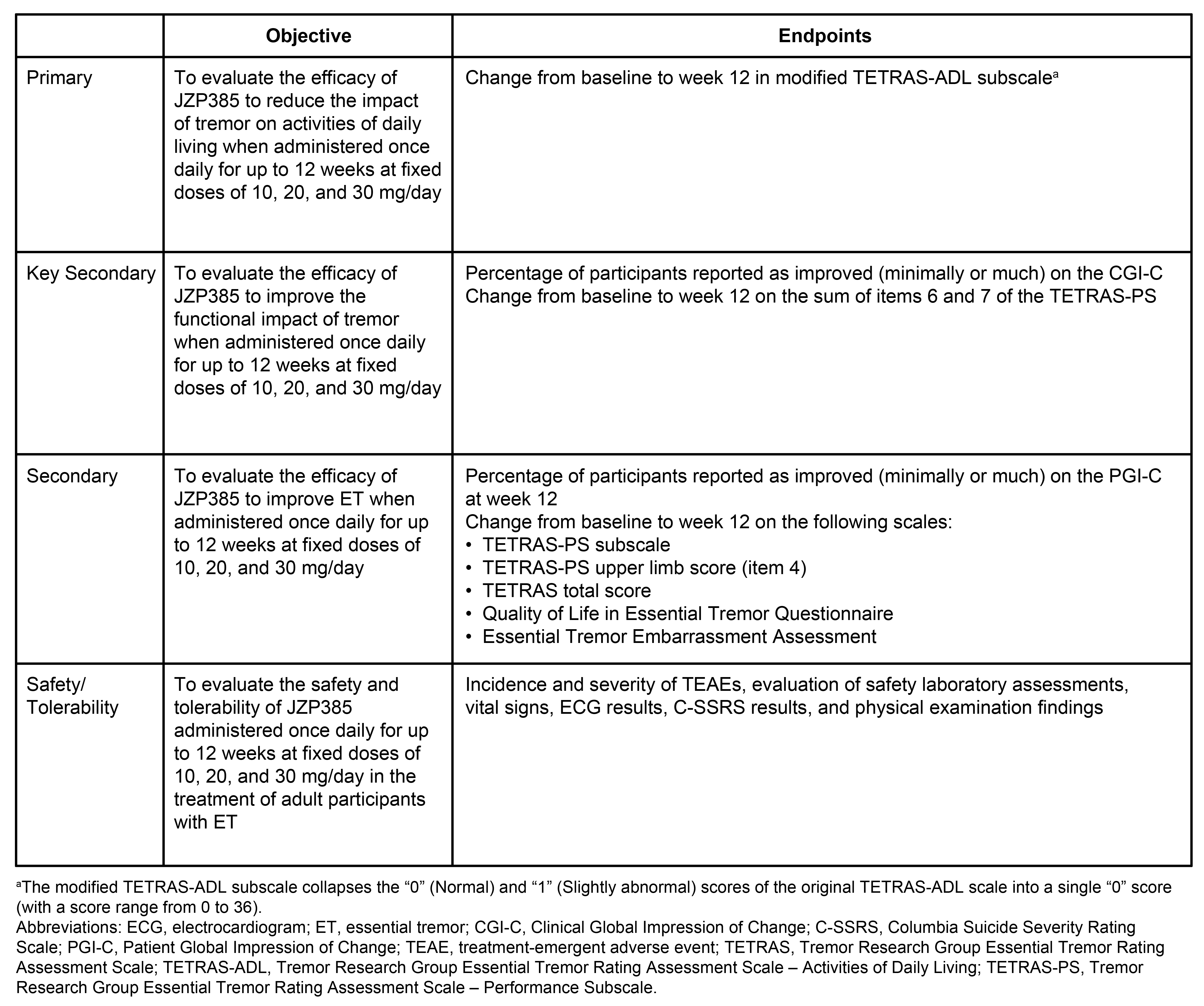
Method: This 12-week, double-blind, placebo-controlled, randomized, parallel-group, multicenter study will recruit adults diagnosed with ET per the MDS Consensus Statement on the Classification of Tremors [4] and experiencing moderate to severe disability (Tremor Research Group Essential Tremor Rating Assessment Scale–Activities of Daily Living [TETRAS-ADL] score of ≥22, TETRAS-Performance Subscale items 6 and 7 combined score of >5, and Clinical Global Impression of Severity rating of at least “moderate”). Following a screening and ET-drug washout period of ≤7 weeks, participants will be randomized (1:1:1:1) to titrated doses of 10, 20, or 30 mg JZP385 QD or placebo. Randomization will be stratified by TETRAS-ADL score severity (≤27 and >27). The primary endpoint is the change from baseline to week 12 in a modification of TETRAS-ADL score (Table). Secondary endpoints will evaluate other efficacy measures and safety/tolerability.
Results: With a target enrollment of 400 participants (320 evaluable), this 12-week study is powered to detect a 3.2-point change on the modified TETRAS-ADL comparing each dose level of JZP385 to placebo (common σ=6.2, two-sided α=0.05). Enrollment is planned to begin in late 2021.
Conclusion: The goal of this trial is to further examine the potential efficacy and safety of JZP385 for the treatment of moderate to severe ET. The study design seeks to overcome limitations of prior research and build upon the proof-of-concept study by moving beyond measures of tremor amplitude to focus on more clinically relevant assessments of functional impairment.
References: 1. Louis ED, et al. Correlates of functional disability in essential tremor. Mov Disord. 2001;16(5):914-20. 2. Haubenberger D, Hallett M. Essential tremor. N Engl J Med. 2018;378(19):1802-10. 3. Diaz NL, Louis ED. Survey of medication usage patterns among essential tremor patients: movement disorder specialists vs. general neurologists. Parkinsonism Relat Disord. 2010;16(9):604-7. 4. Bhatia KP, et al. Consensus Statement on the classification of tremors from the Task Force on Tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33(1):75-87.
To cite this abstract in AMA style:
A. Sterkel, W. Ondo, R. Elble, P. Lewitt, R. Pahwa, C. Urrea, L. Huang, L. Hickey, M. Baladi. A phase 2b randomized, double-blind, placebo-controlled study of suvecaltamide in the treatment of adults with moderate to severe essential tremor: study design and methodology [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/a-phase-2b-randomized-double-blind-placebo-controlled-study-of-suvecaltamide-in-the-treatment-of-adults-with-moderate-to-severe-essential-tremor-study-design-and-methodology/. Accessed February 5, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-phase-2b-randomized-double-blind-placebo-controlled-study-of-suvecaltamide-in-the-treatment-of-adults-with-moderate-to-severe-essential-tremor-study-design-and-methodology/