Category: Parkinson’s Disease: Clinical Trials
Objective: To further evaluate the efficacy of foslevodopa/foscarbidopa (LDp/CDp), a soluble formulation of levodopa/carbidopa (LD/CD) prodrugs delivered by 24 hour/day customizable continuous subcutaneous infusion, for people with advanced Parkinson’s disease (PwaPD).
Background: Analyzing treatment impact by effect size, number needed to treat (NNT), and percent change improves understanding of the clinical and practical significance of an intervention’s efficacy, supporting informed and patient-centric healthcare decisions.
Method: PwaPD with ≥ 2.5 hours “Off” time/day were enrolled in 2 phase 3 trials investigating LDp/CDp (range: approximately 700−4260 mg LD equivalents/day) [1, 2]. PwaPD were randomized 1:1 to LDp/CDp or oral immediate-release (IR) LD/CD in the 12-week double-blind study (NCT04380142) [2]; in the 52-week study all PwaPD received open-label LDp/CDp (NCT03781167) [2]. Effect size, NNT, and percent change from baseline (BL) were evaluated post hoc for “Off”/“On” times (Parkinson’s Disease [PD] diary), daily activities (Movement Disorder Society-Unified Parkinson’s Disease Rating Scale Part II), sleep (PD Sleep Scale-2), and quality of life (39-item PD Questionnaire).
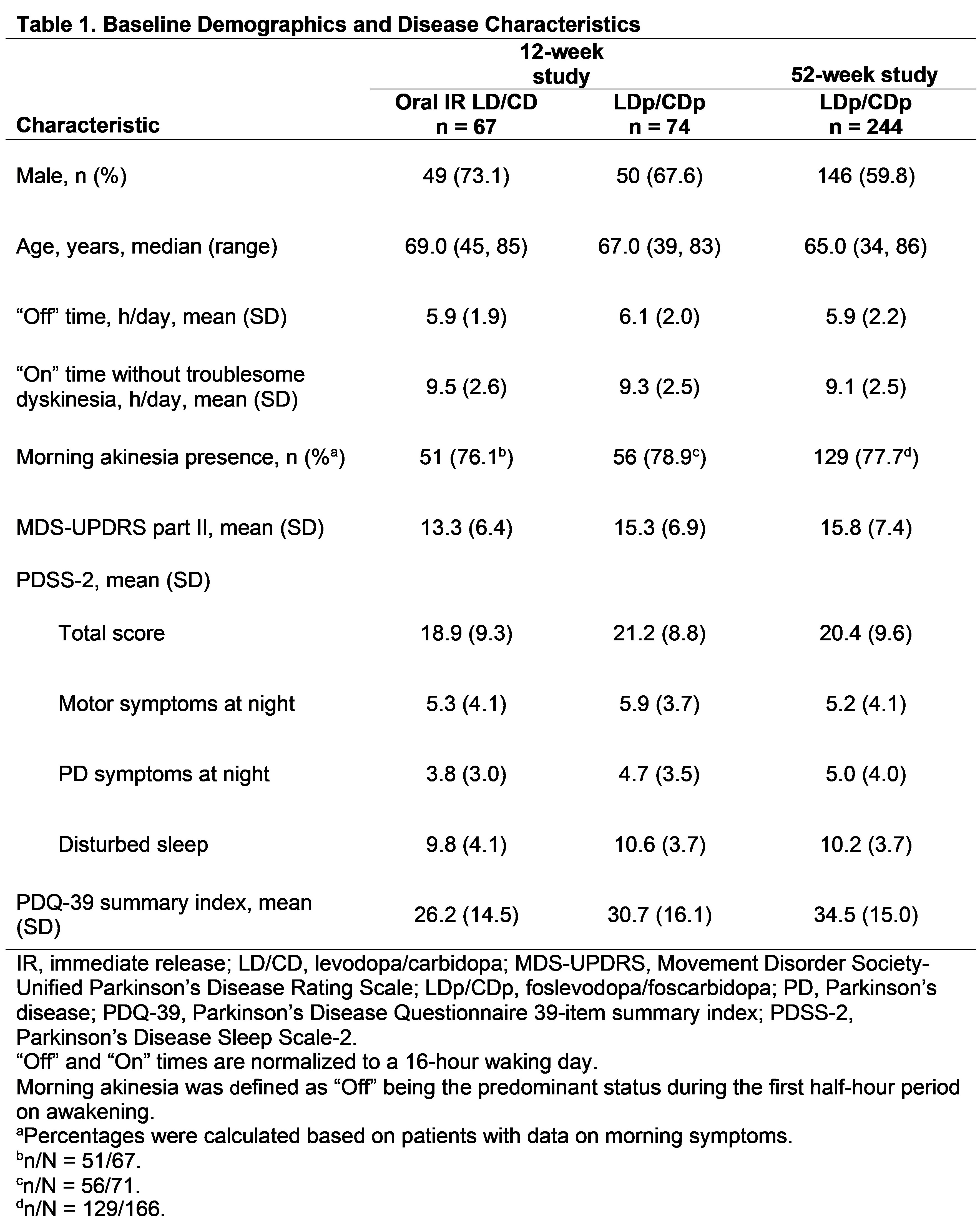
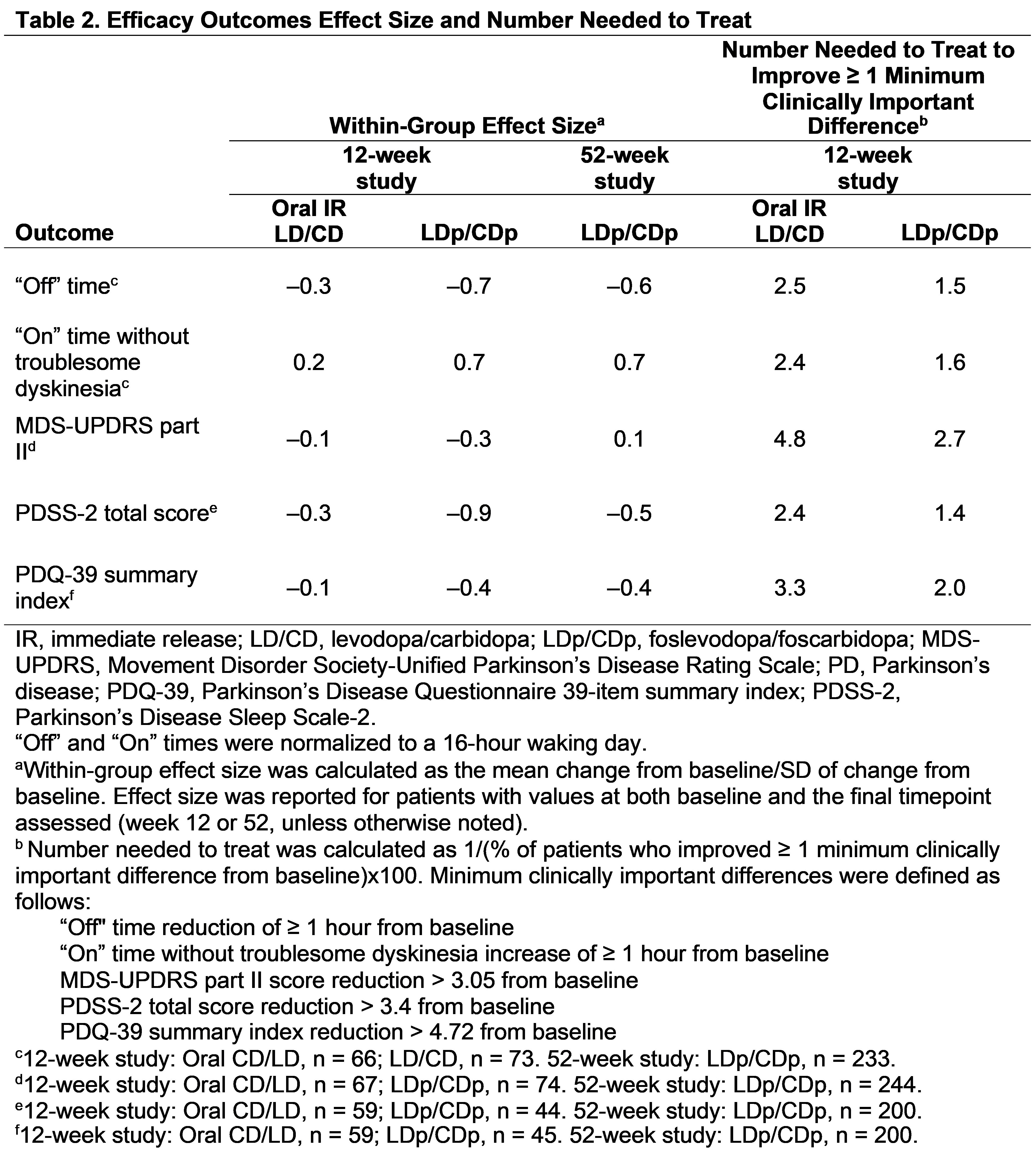
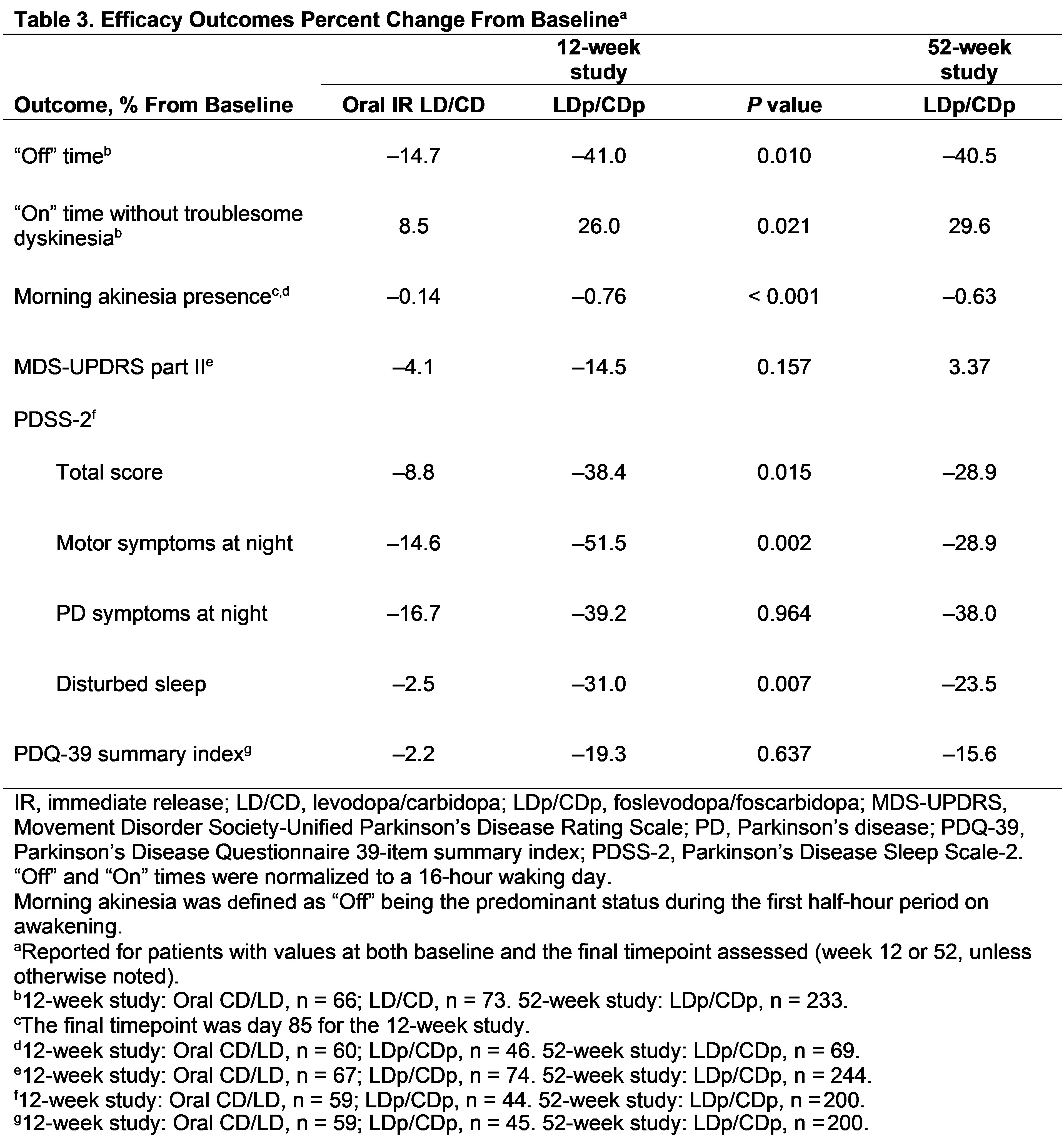
Results: This analysis includes 141 PwaPD from the 12-week study (oral IR LD/CD, n = 67; LDp/CDp, n = 74), and 244 PwaPD (LDp/CDp) from the 52-week study [table1]. Baseline characteristics were generally similar across studies. Within-group effect size showed a benefit of LDp/CDp vs oral IR LD/CD at week 12 for reduction in “Off” time (–0.7 vs –0.3) and improvements in “On” time without troublesome dyskinesia (0.7 vs 0.2); LDp/CDp effect sizes were similar at weeks 12 and 52 [table2]. At week 12, the NNT for minimum clinically important differences in “Off” time (reduced ≥ 1 hour) and “On” time without troublesome dyskinesia (improved ≥ 1 hour) was lower with LDp/CDp (1.5 and 1.6) vs oral IR LD/CD (2.5 and 2.4). Percent change, effect size, and NNT analyses generally showed a benefit for LDp/CDp vs oral IR LD/CD in improving motor and nonmotor symptoms [table2,3].
Conclusion: In PwaPD, LDp/CDp demonstrated improved motor function, daily activities, sleep, and quality of life vs PwaPD on oral IR LD/CD when assessed by effect size, NNT, and percent change from BL. These findings underscore the benefit of LDp/CDp and may facilitate more informed treatment decisions by PwaPD and healthcare providers.
Table 1
Table 2
Table 3
References: 1. Soileau MJ, et al. Lancet Neurol. 2022;21:1099–1109.
2. Aldred J, et al. Neurol Ther. 2023;12:1937–1958.
To cite this abstract in AMA style:
M. Soileau, M. Bouchard, I. Malaty, J. Parra, L. Bergmann, R. Gupta, A. Spiegel, E. Freire-Alvarez. A Post Hoc Efficacy Analysis of Phase 3 Trials of Continuous Subcutaneous Foslevodopa/Foscarbidopa in Patients With Parkinson’s Disease [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/a-post-hoc-efficacy-analysis-of-phase-3-trials-of-continuous-subcutaneous-foslevodopa-foscarbidopa-in-patients-with-parkinsons-disease/. Accessed February 14, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-post-hoc-efficacy-analysis-of-phase-3-trials-of-continuous-subcutaneous-foslevodopa-foscarbidopa-in-patients-with-parkinsons-disease/