Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate the long-term effect of prasinezumab on motor progression, assessed as change in MDS-UPDRS Part III in OFF- and ON-state, and MDS-UPDRS Part II scores over 4 years from the start of the trial.
Background: The PASADENA study is an ongoing Phase II, multicenter, randomized, double-blind, placebo-controlled trial evaluating the safety and efficacy of intravenous prasinezumab, administered every 4 weeks, in early-stage Parkinson’s disease (PD) [1]. During the double-blind study period, prasinezumab-treated individuals showed less progression of motor signs (MDS-UPDRS Part III) [1].
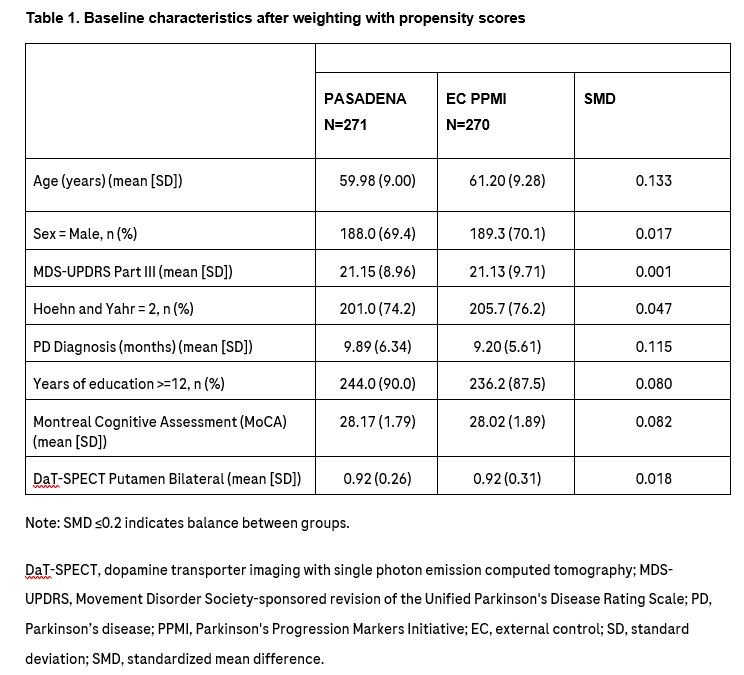
Method: In lack of a long-term placebo arm, we compared participants enrolled in the PASADENA open-label extension (OLE) to an external comparison arm derived from the Parkinson’s Progression Markers Initiative (PPMI) observational study [Table 1] [2].
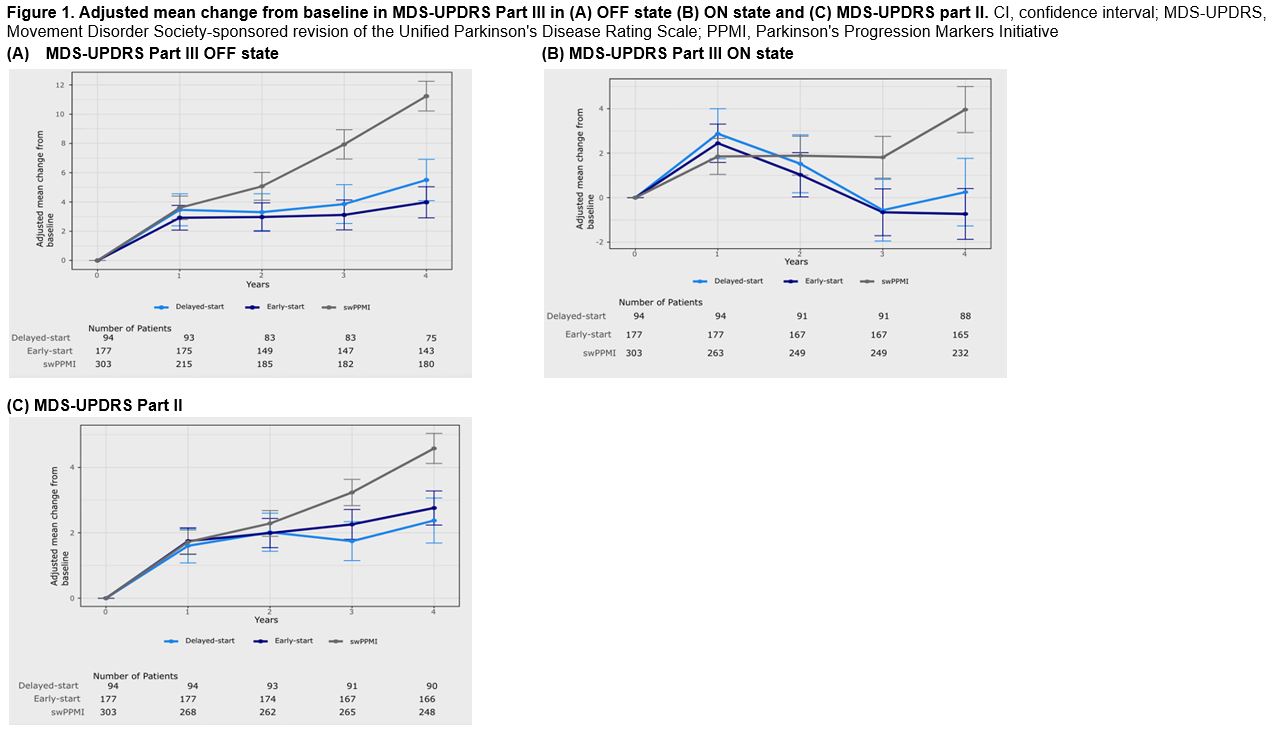
Results: Both PASADENA delayed- (n=94) and early-start (n=177) groups showed a slower decline (less increase in score) on MDS-UPDRS Part III in OFF- and ON-state and on MDS-UPDRS Part II, compared to the PPMI external comparison (n=303) [Figure 1].
Conclusion: This exploratory analysis, which requires confirmation in further studies, suggests that prasinezumab might slow motor progression long-term in PD.
Table 1
Figure 1
References: [1] Pagano, G., et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N Engl J Med 387, 421-432 (2022).
[2] Marek, K., et al. The Parkinson’s progression markers initiative (PPMI) – establishing a PD biomarker cohort. Ann Clin Transl Neurol 5, 1460-1477 (2018)
To cite this abstract in AMA style:
G. Pagano, A. Monnet, A. Reyes, B. Ribba, H. Svoboda, T. Kustermann, T. Simuni, R. Postuma, N. Pavese, F. Stocchi, K. Brockmann, K. Smigorski, V. Gerbaldo, P. Fontoura, R. Doody, G. Kerchner, P. Brundin, K. Marek, A. Bonni, T. Nikolcheva. Effect of Prasinezumab on Parkinson’s Disease Motor Progression in a Long-term Open-label Extension of the PASADENA Trial [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/effect-of-prasinezumab-on-parkinsons-disease-motor-progression-in-a-long-term-open-label-extension-of-the-pasadena-trial/. Accessed February 11, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/effect-of-prasinezumab-on-parkinsons-disease-motor-progression-in-a-long-term-open-label-extension-of-the-pasadena-trial/