Category: Huntington's Disease
Objective: To prove the efficacy and safety of dextromethorphan/quinidine 20mg/10mg (Nuedexta®) in patients with irritability due to HD.
Background: Irritability is one of HD’s most common neuropsychiatric symptoms, often leading to aggressive behavior with severe consequences. Currently, no medications are approved to treat specifically irritability in HD, and the available treatments are off–label and imperfect. Therefore, an effective medication to address this disruptive behavioral symptom that can destroy patient/caregiver relationships and negatively impact the quality of life is imperative. We hypothesize that Nuedexta®, a medication approved by the FDA in 2010 to treat pseudobulbar affect or exaggerated emotional expression incongruent to mood due to an underlying brain disorder, will decrease irritability in individuals with HD and minimize aggression and outbursts.
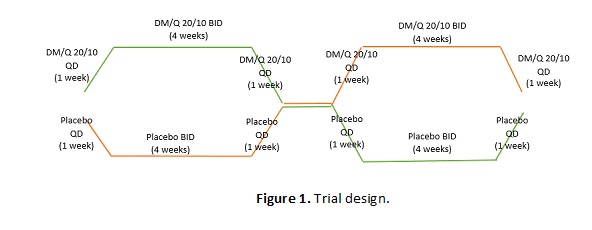
Method: Randomized, double-blind, placebo-controlled, crossover, proof of concept study with 20 adults with verified HD mutation and irritability [defined as an Irritability Scale (IS) score > 14], as described in Figure 1 [Figure 1]. Primary outcomes: improvement of irritability, described as a 4-point change from baseline in the IS. Secondary outcomes: Irritability, as quantified by the changes in the total IS score; Change of behavioral symptoms; Change in the motor symptoms; Change in functional independence.
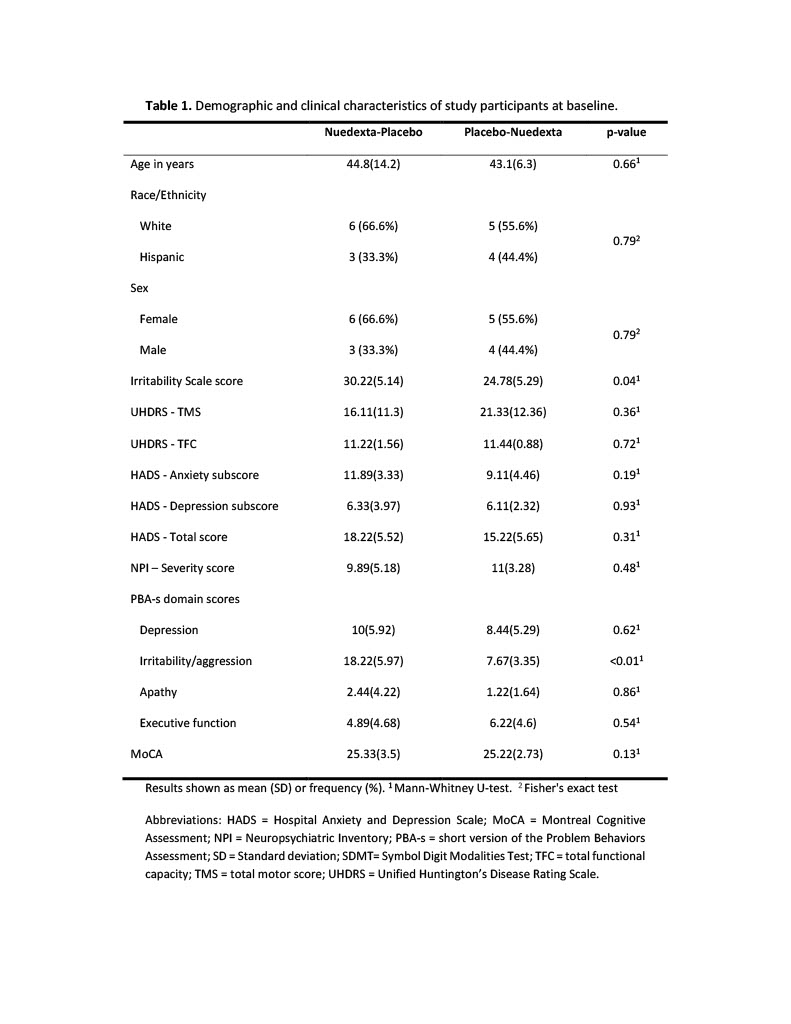
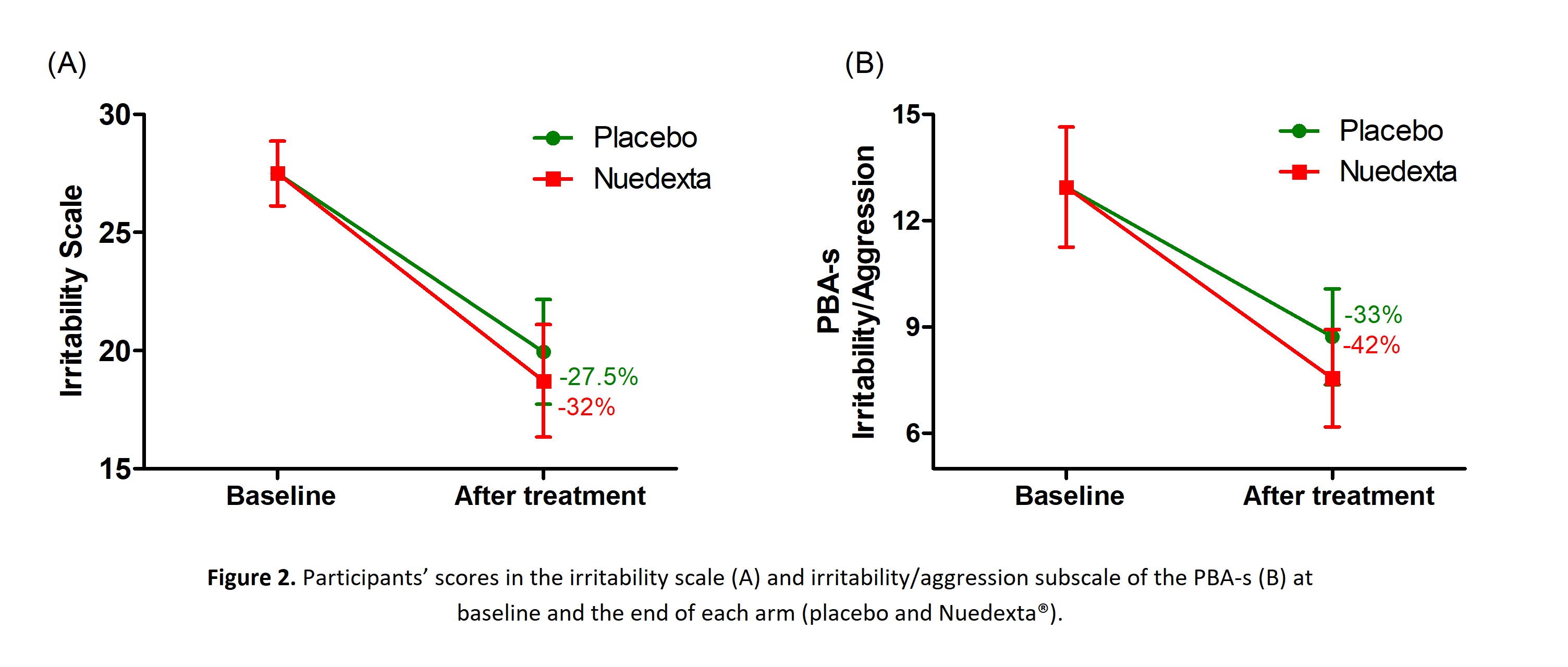
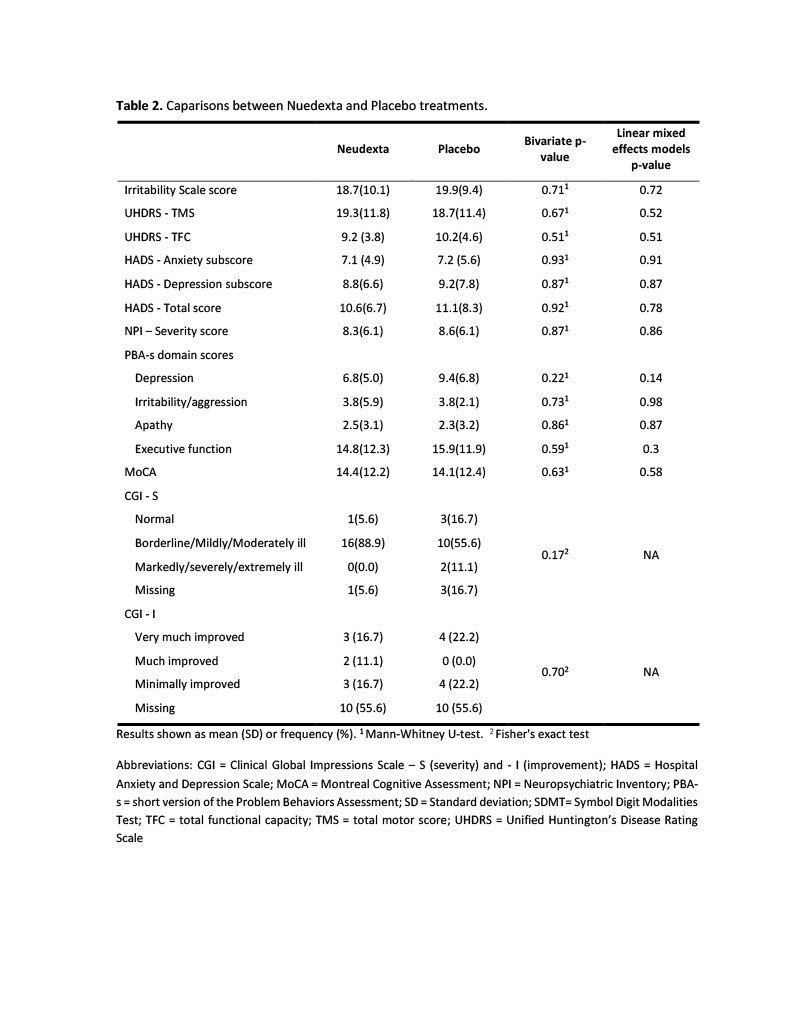
Results: 18 individuals completed the trial, and a general description of the study population is provided in Table 1 [Table 1]. Participants were divided into two groups according to the treatment order. We found significant differences between groups in the scores on the IS and PBA-s irritability/aggression at baseline (Table 1). We observed a significant reduction in the IS score with both placebo and Nuedexta® treatments (Figure 2A). The results were similar when we analyzed the irritability/aggression score of the PBA-s (Figure 2B) [Figure 2]. In addition, we did not find differences between treatments for any of the secondary outcomes, as described in Table 2 [Table 2].
Conclusion: Although we observed a pronounced decrease in the IS score after the treatment with Nuedexta® (mean reduction of 8.8 points, or 32% compared to the baseline), our study revealed a strong placebo effect (mean decrease of 7.6 points, 27.5% with the placebo treatment) and there was no difference between treatments.
Funding: Cures Within Reach
To cite this abstract in AMA style:
E. Furr Stimming, S. Abdollah Zadegan, J. Patino, N. Rocha. Evaluating the efficacy of dextromethorphan/quinidine (DM/Q) in treating irritability in Huntington’s disease [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/evaluating-the-efficacy-of-dextromethorphan-quinidine-dm-q-in-treating-irritability-in-huntingtons-disease/. Accessed February 11, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/evaluating-the-efficacy-of-dextromethorphan-quinidine-dm-q-in-treating-irritability-in-huntingtons-disease/