Category: Parkinson’s Disease: Clinical Trials
Objective: To assess nocturia over time and its correlation with quality of life (QoL) in patients with Parkinson’s disease (PD) treated with foslevodopa/foscarbidopa (LDP/CDP).
Background: As PD progresses, patients experience worsening nocturia and increased fall risk when getting up at night. Dopaminergic influences on D1 receptors may underlie nocturia symptoms. Continuous dopaminergic delivery can improve sleep and nocturia in PD [1,2]. LDP/CDP provides continuous (24‑hour/day) subcutaneous infusion (CSCI) of carbidopa/levodopa (CD/LD) prodrugs.
Method: Data from a 12-week (wk), phase 3, randomized, double‑blind trial evaluating LDP/CDP vs oral immediate-release CD/LD in patients with advanced PD (NCT04380142) and from a 52-wk, phase 3, single-arm, open-label safety study evaluating LDP/CDP in patients with PD (NCT03781167) were included in this post hoc analysis. A mixed effects regression model, and analysis of covariance were used to analyze mean changes in nocturia (item 8 scores from the Parkinson’s Disease Sleep Scale-2 [PDSS-2]) over time. Spearman’s correlation coefficients were used for correlation analyses between PDSS-2 and QoL (39-item Parkinson’s Disease Questionnaire [PDQ-39]) at baseline (BL) and change from BL to wk 12 or wk 52.
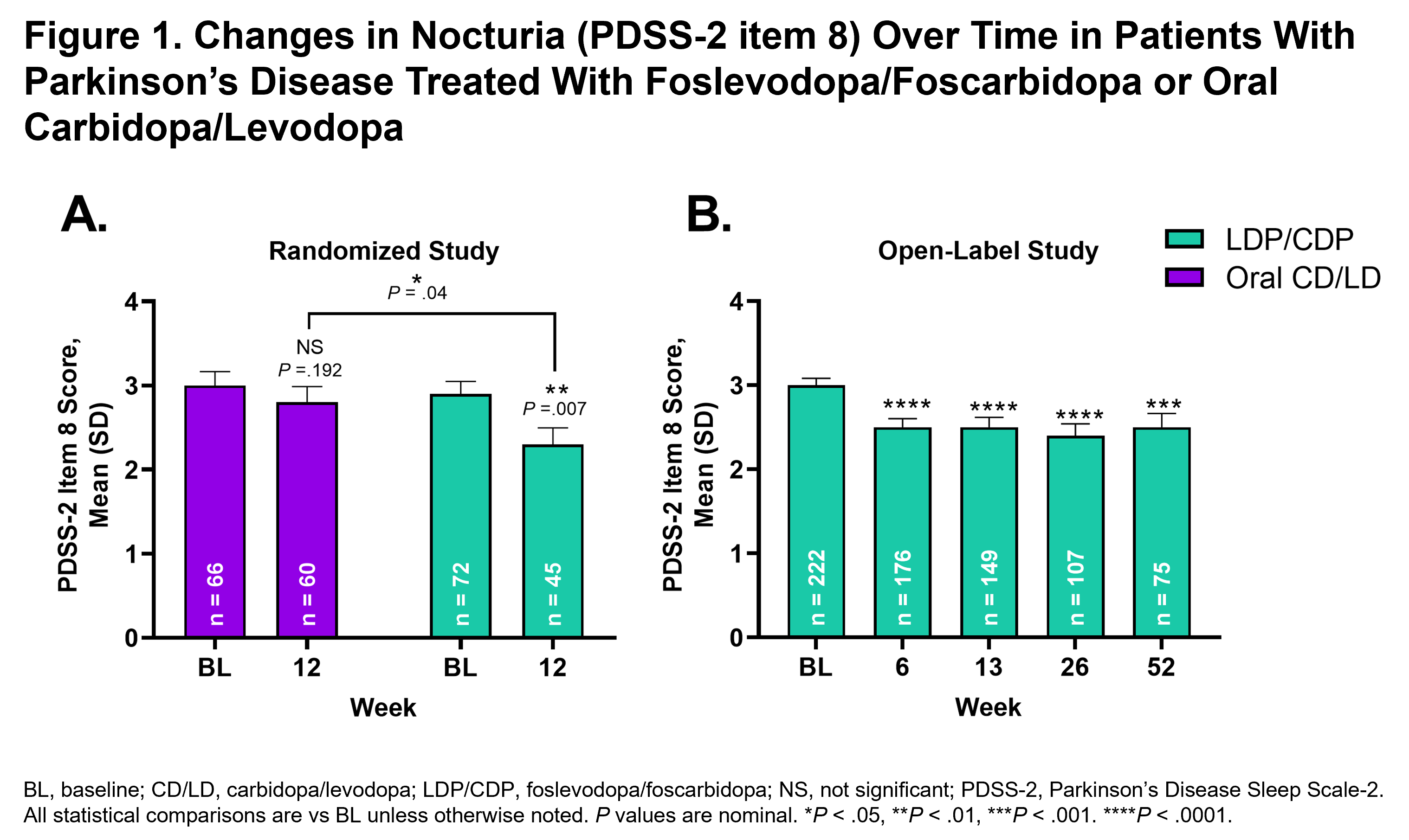
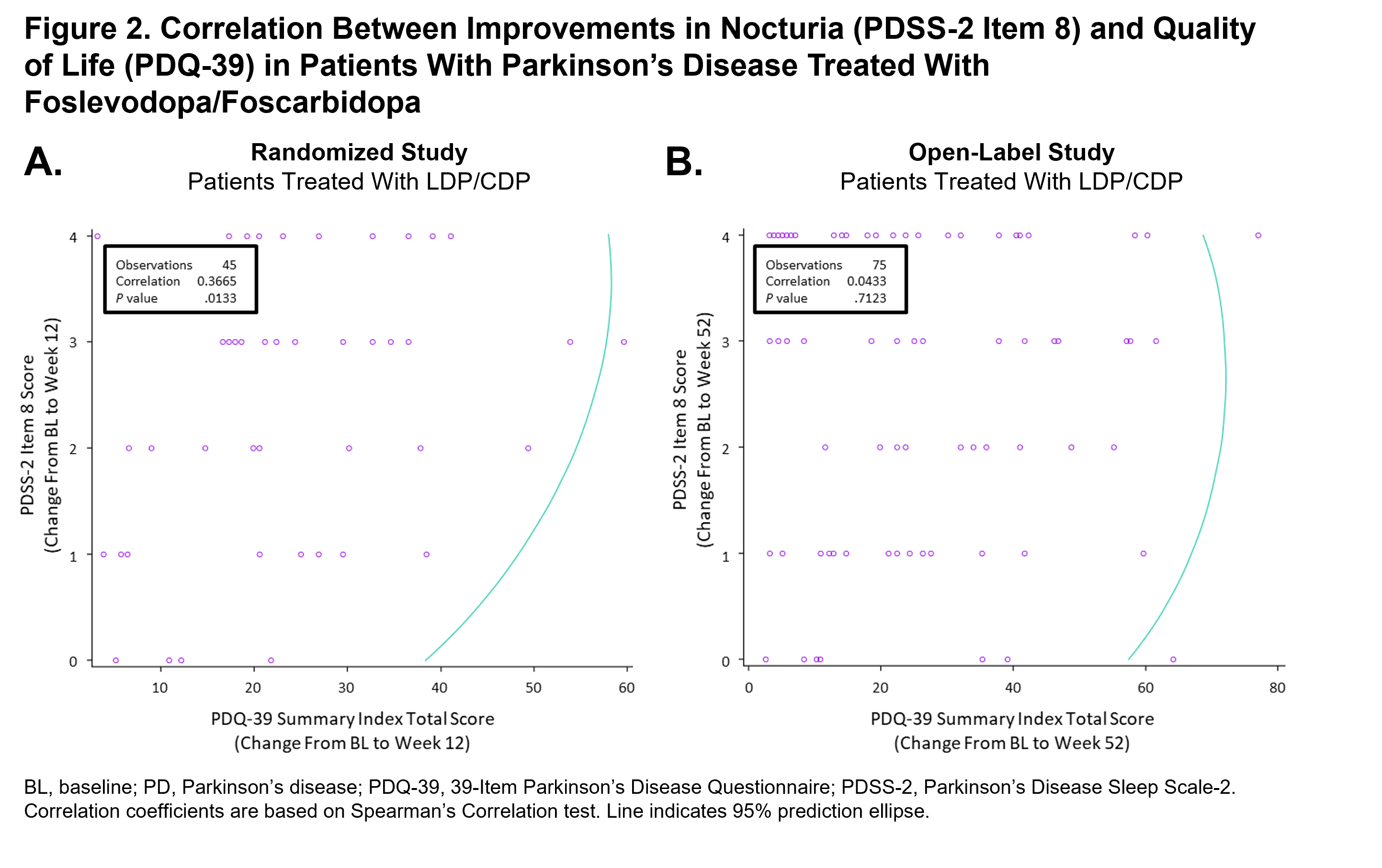
Results: In the randomized study, treatment with LDP/CDP led to a significant improvement in nocturia symptoms (reduction in mean PDSS-2 item 8 score) from BL to wk 12 (P<.01); an improvement that was significantly greater when compared with patients treated with CD/LD (P<.05) [figure 1]. In the open-label study, there was also significant reduction in the mean PDSS-2 item 8 score at wks 6, 13, 26, and 52, compared with BL (P<.001 for all comparisons). Previously published results have also demonstrated improvement in PDQ-39 with LDP/CDP in patients with PD [3]. PDSS-2 scores at BL and improvements in PDSS-2 item 8 and PDQ-39 scores were significantly positively correlated at wk 12 for the LDP/CDP group in the randomized study (BL, rho=0.28, P=.02; wk 12, rho=0.37, P=.01) [figure 2], but were not significantly correlated at wk 52 (BL, rho=0.13, P=.06; wk 52, rho=0.04, P=.71) in the open-label study.
Conclusion: Use of LDP/CDP CSCI in patients with PD leads to reductions in patient-reported symptoms of nocturia that may be correlated with improvement in QoL.
References: 1. Martinez-Martin P, et al. J Parkinsons Dis. 2011;1(2):197−203.
2. Chaudhuri KR, et al. Parkinsonism Relat Disord. 2013;19(7):660−665.
3. Soileau MJ, et al. Lancet Neurol. 2022;21:1099–1109.
To cite this abstract in AMA style:
K. Chaudhuri, M. Bouchard, E. Freire-Alvarez, R. Pahwa, L. Bergmann, R. Gupta, P. Kukreja, M. Shah, S. Isaacson. Improvement of nocturia symptoms and associated quality of life in patients with Parkinson’s disease treated with foslevodopa/foscarbidopa: results from 2 phase 3 trials [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/improvement-of-nocturia-symptoms-and-associated-quality-of-life-in-patients-with-parkinsons-disease-treated-with-foslevodopa-foscarbidopa-results-from-2-phase-3-trials/. Accessed February 8, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/improvement-of-nocturia-symptoms-and-associated-quality-of-life-in-patients-with-parkinsons-disease-treated-with-foslevodopa-foscarbidopa-results-from-2-phase-3-trials/