Category: Technology
Objective: A metadata framework for Digital Health Technologies (DHT) to support enhanced regulatory maturity, and to facilitate standardization and harmonization of data collection
Background: DHTs could complement traditional clinical assessments in PD through high frequency data collection, greater accuracy, improved objectivity, and capturing fluctuating symptoms and occasional events eg freezing of gait. But poor comparability of data from DHTs (eg between devices, between studies) is a barrier to widespread adoption and regulatory acceptability.
Method: A key requirements for technology adoption is a framework to define and describe data collection for both primary measurements and metadata. We focus on the metadata required to describe DHTs in the broader clinical trial context, along with concerns about DHTs expressed in meetings with FDA and EMA.
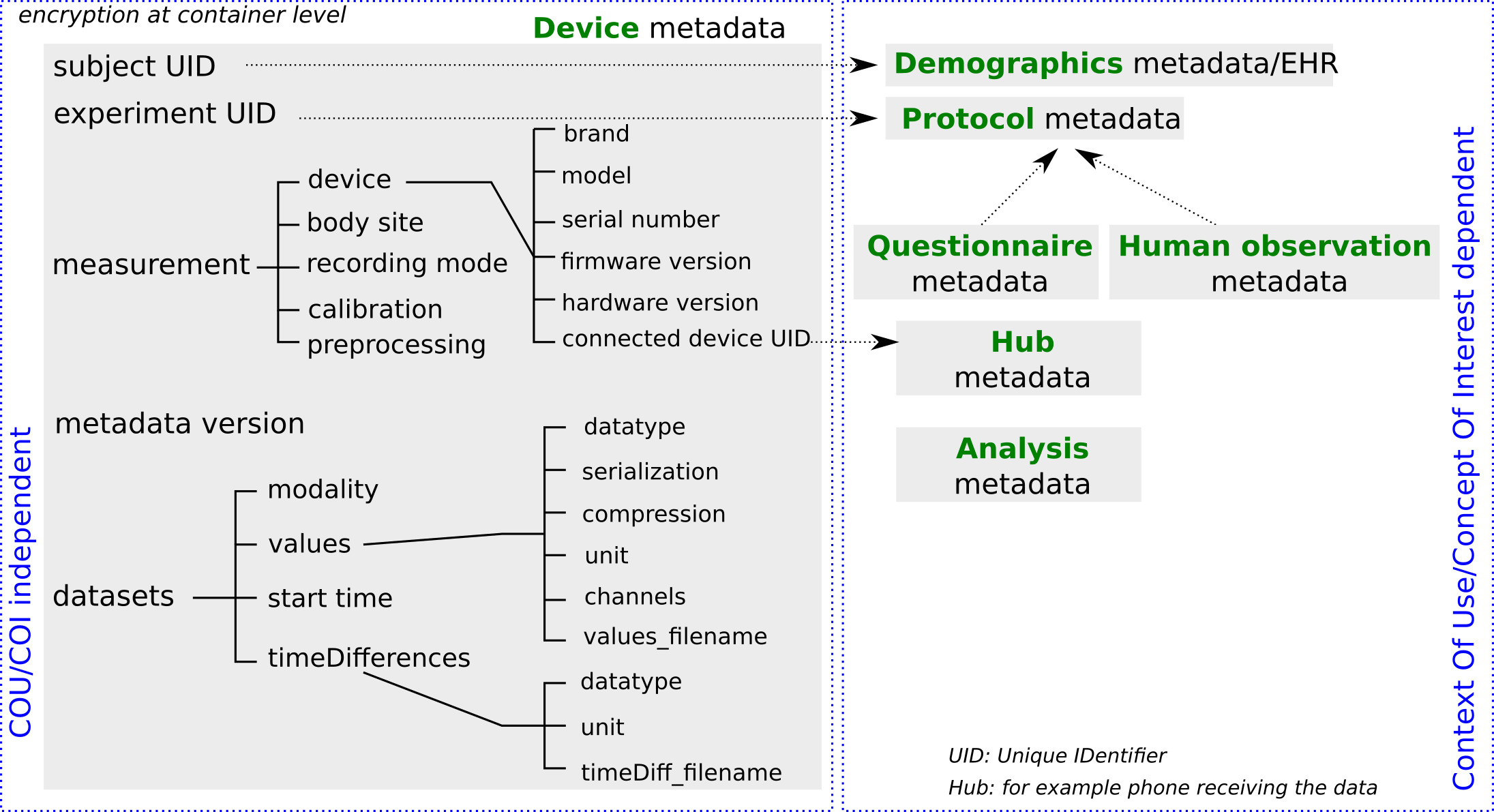
Results: Our proposed framework divides metadata into (1) device metadata comprising metadata about the sensors and the measurement tool, and (2) clinical trial concept of interest (COI) metadata. The device metadata are quite generic – and can be used to describe data collected for many COIs eg: an accelerometer could be used to measure many different parameters in various patient populations, including total activity, gait speed, turning gait, falls, sleep, and tremor. For all these applications, there is an additional need for COI metadata to complement this core. The framework illustrated below has been applied to several use cases (see Figure)
Conclusion: We propose a metadata framework to help address barriers to the adoption and greater impact of DHTs in PD drug development: it supports pre-specification of the minimum required values of relevant metadata fields, and by comparing pre-specified with actual values, to provide a quality assurance framework for DHTs.
The framework proposes that the COI metadata and the pre-specified values of both device and COI metadata (where applicable) are documented in a dedicated DHT Charter that describes how DHT data should be collected.
The proposed metadata framework captures the information needed to optimise the value of the DHT derived measures, and by means of pre-specification, provides a way to standardize and quality assure collected DHT data. It is a step forward towards harmonization of data collection across DHTs and studies.
To cite this abstract in AMA style:
D. Hill, D. Stephenson, J. Brayanov, K. Claes, R. Badawy, S. Sardar, K. Fisher, S. Lee, A. Bannon, V. Terebaite, R. Bhatnagar. Metadata standards to support deployment of Digital Health Technologies in Clinical Trials in Parkinson’s Disease (PD) [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/metadata-standards-to-support-deployment-of-digital-health-technologies-in-clinical-trials-in-parkinsons-disease-pd/. Accessed March 3, 2026.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/metadata-standards-to-support-deployment-of-digital-health-technologies-in-clinical-trials-in-parkinsons-disease-pd/