Objective: Evaluate the pharmacokinetics (PK), safety, and tolerability of a once-daily (QD) suvecaltamide (JZP385) formulation and its major active metabolites (JZZ05000034=M01, JZZ05000035=M02) in a Phase 1, double-blind, parallel design, within-participant, multiple ascending dose study.
Background: Suvecaltamide, a highly-selective CaV3 channel modulator, is currently in Phase 2 development to treat essential tremor (NCT05122650) and residual Parkinson’s disease tremor (NCT05642442).
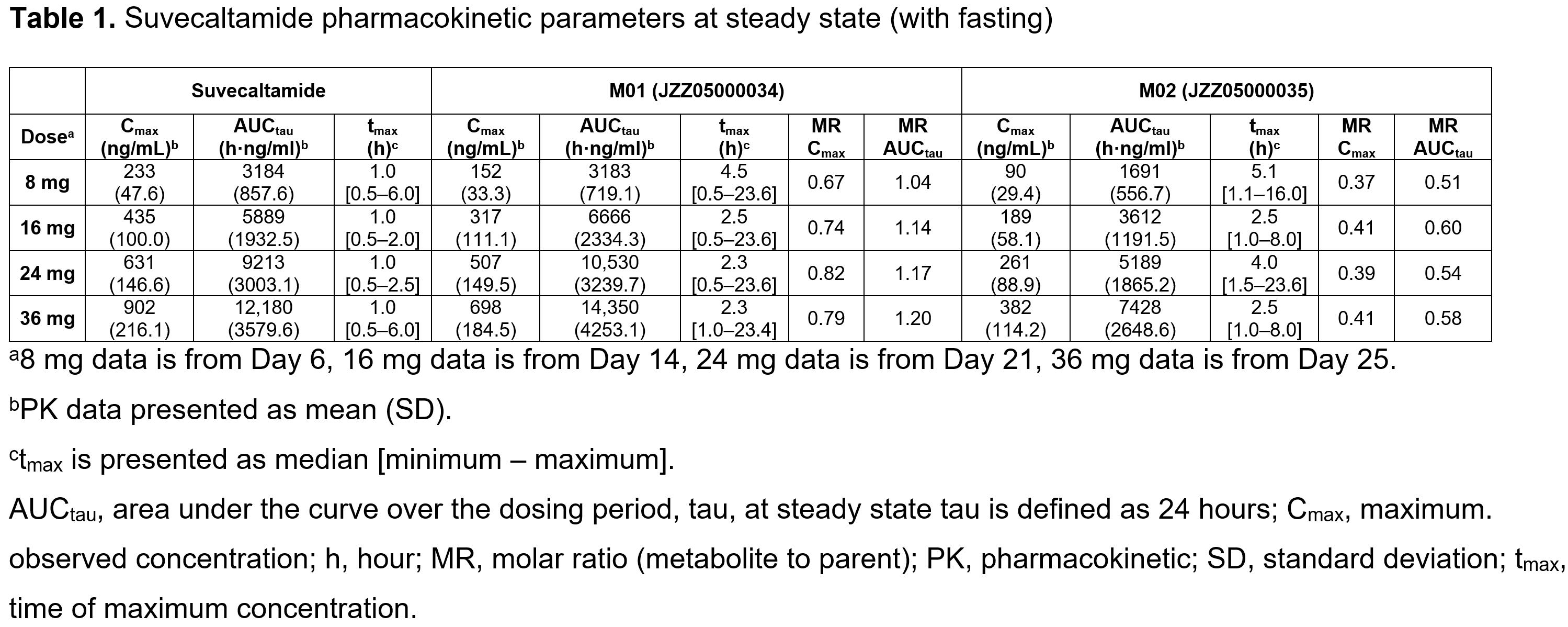
Method: Healthy participants (18‒75 years) were randomized to suvecaltamide or placebo (Part 1: 8 mg QD, days 1‒7; Part 2: 8 mg QD, days 1‒7; 16 mg QD, days 8‒14; 24 mg QD, days 15‒21; 36 mg QD, days 22‒25). PK, time-matched electrocardiograms, treatment-emergent adverse events (TEAEs), and food-effect data were collected.
Results: Participants (suvecaltamide: n=20/formulation/part; placebo: n=10/part) were similar in age, sex, race, and baseline characteristics across treatment groups and study parts. Fasted, steady-state PK parameters for suvecaltamide and its major active metabolites were approximately dose proportional [table 1]. A high-fat/high-calorie meal had negligible effect on suvecaltamide Cmax and AUC, but prolonged tmax. For all doses, the upper bound 90% CI of the placebo-corrected change from baseline in Fridericia’s corrected QT interval at each analyte’s mean Cmax was substantially below the 10-msec regulatory threshold of concern. Across study parts, the most common TEAEs were dizziness (suvecaltamide, 25%; placebo, 10%), insomnia (suvecaltamide, 20%; placebo, 20%), and headache (suvecaltamide, 18%; placebo, 30%). Most TEAEs were mild to moderate in severity, and their incidence tended to decrease with increasing dose.
Conclusion: Results from this Phase 1 study expand our understanding of suvecaltamide PK, safety, and tolerability, supporting further clinical development of the QD formulation of suvecaltamide.
Table 1
To cite this abstract in AMA style:
S. Markova, M. Baladi, M. Lee, C. Chen. Once-Daily Suvecaltamide Pharmacokinetics, Safety, & Tolerability–Phase 1, Randomized, Double-Blind, Multiple Ascending Dose Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/once-daily-suvecaltamide-pharmacokinetics-safety-tolerability-phase-1-randomized-double-blind-multiple-ascending-dose-study/. Accessed January 27, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/once-daily-suvecaltamide-pharmacokinetics-safety-tolerability-phase-1-randomized-double-blind-multiple-ascending-dose-study/