Category: Parkinson’s Disease: Clinical Trials
Objective: Review available efficacy data from Phase 3 studies assessing continuous subcutaneous apomorphine infusion (CSAI) for patients with PD experiencing motor fluctuations.
Background: Despite decades of real-world experience, only two Phase 3 studies have been performed to evaluate the safety and efficacy of CSAI for the management of motor fluctuations in PD. The TOLEDO [1] and InfusON studies enrolled similar patient populations and used the same assessments for consistent evaluation of efficacy.
Method: The TOLEDO study (NCT02006121; N=107) was a randomized, double-blind, placebo-controlled, 12-week trial conducted in Europe (23 sites), followed by a 52-week open-label Extension phase [1],[2]. InfusON (NCT02339064; N=99) is an open-label trial conducted in the US (19 sites), consisting of a Titration period and a 52-week Maintenance period. Both trials enrolled patients experiencing motor fluctuations despite optimized levodopa plus current or prior use of ≥1 other therapy. The primary endpoint for both trials was change from Baseline in daily OFF time at Study Week 12 (TOLEDO) or Maintenance Period Week 12 (InfusON).
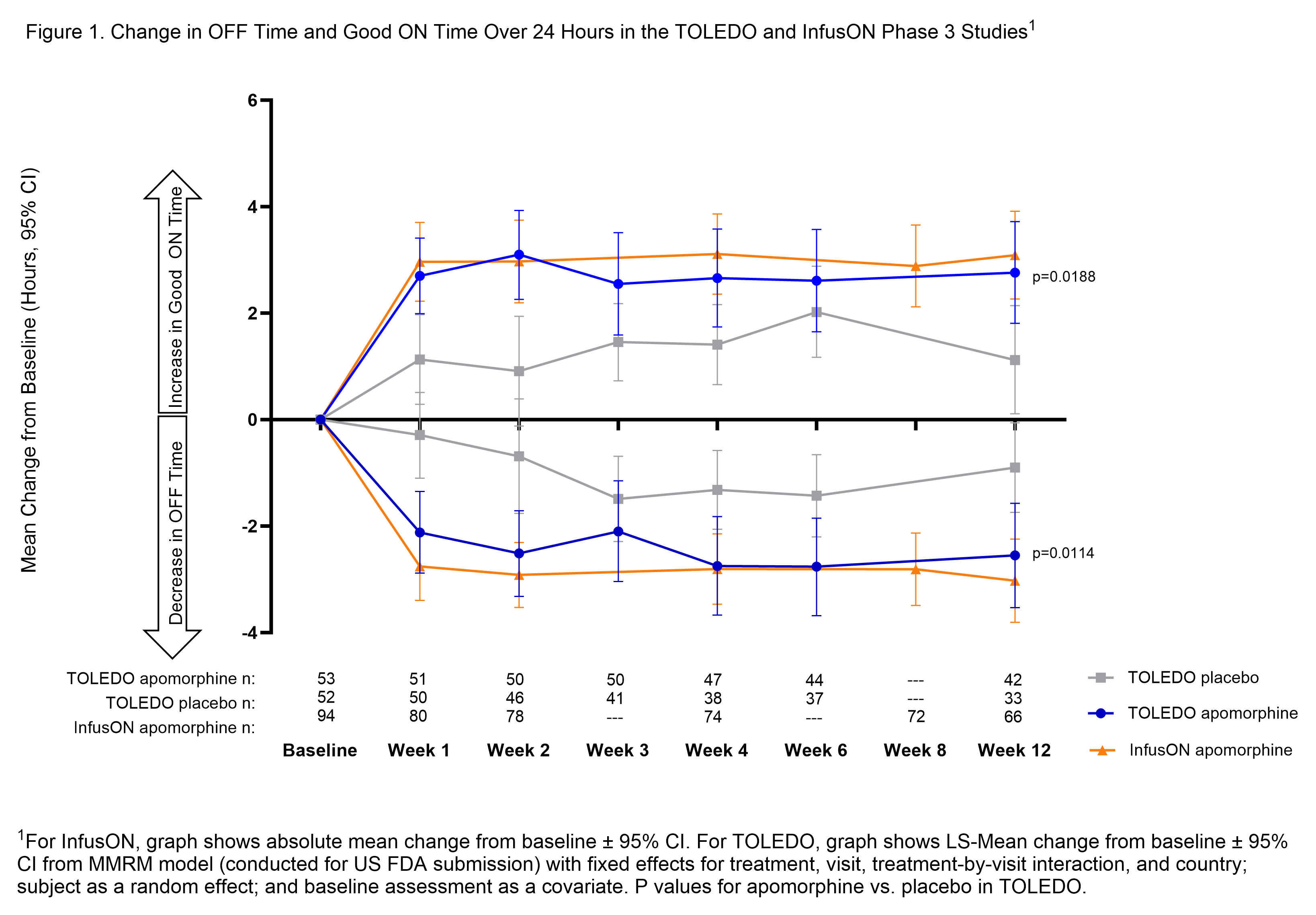
Results: Patients entering each trial were experiencing >6 hours of mean daily OFF time. Reductions in OFF time with CSAI were statistically significant vs. placebo in TOLEDO, and highly consistent between trials at Week 12 (-2.6 h Toledo; -3.0 h InfusON) and mirrored by improvements in Good ON time (2.8 h Toledo; 3.1 h InfusON) [figure1]. This improvement was meaningful to patients with the majority reporting improvement in overall health status on Patient Global Impression relative to baseline (79% TOLEDO; 89% InfusON). CSAI efficacy was maintained through Week 52 (OFF time reductions: -3.7 h Toledo and -3.2 h InfusON; Good ON time improvement: 3.3 h TOLEDO and 3.7 InfusON from original study baseline). The safety and tolerability profile of CSAI was also generally consistent across both studies, with the most common treatment-emergent adverse event being infusion site nodules (over 50% of patients) that were typically mild to moderate in severity and seldom led to discontinuation.
Conclusion: Despite differences in study design, results from both Phase 3 studies showed consistent, reproducible efficacy in reducing OFF time and increasing Good ON time from baseline values that were maintained over at least 1 year.
Changes in OFF Time and Good ON Time
References: [1] Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, Henriksen T, van Laar T, Spivey K, Vel S, Staines H, Lees A. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurology 17; (2018) 749-759.
[2] Katzenschlager R, Poewe W, Rascol O, Trenkwalder C, Deuschl G, Chaudhuri KR, Henriksen T, van Laar T, Lockhart D, Staines H, Lees A. Long-term safety and efficacy of apomorphine infusion in Parkinson’s disease patients with persistent motor fluctuations: Results of the open-label phase of the TOLEDO study. Parkinsonism and Related Disorders 83; (2021) 79–85.
To cite this abstract in AMA style:
R. Pahwa, T. van Laar, K. Dashtipour, G. Ceresoli-Borroni. Overview of Phase 3 Trials of Continuous Subcutaneous Apomorphine Infusion for PD Patients with Motor Fluctuations [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/overview-of-phase-3-trials-of-continuous-subcutaneous-apomorphine-infusion-for-pd-patients-with-motor-fluctuations/. Accessed February 1, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/overview-of-phase-3-trials-of-continuous-subcutaneous-apomorphine-infusion-for-pd-patients-with-motor-fluctuations/