Objective: This post-hoc analysis of Study CTH-301 aimed to assess the safety and efficacy of apomorphine sublingual film (SL-APO) in patients with Parkinson’s disease with or without concurrent dopamine agonists (DA) use at baseline.
Background: In Study CTH-301, SL-APO was generally well tolerated and efficacious over the long term as a treatment for OFF-episodes in patients with Parkinson’s disease.
Method: Study CTH-301 included a dose-optimisation (DO) and long-term safety (LTS) phase. Safety/tolerability assessments included incidence of treatment-emergent adverse events (TEAEs)/DA-related TEAEs, discontinuation rates due to adverse events (AEs), and time to discontinuation due to TEAEs. Efficacy assessments included optimised SL-APO dose, discontinuation rate due to lack of efficacy, changes in Movement Disorder Society-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Part III scores from pre- to post-dose and percentage of patients with a full-ON response within 30 minutes post-dose at weeks 24, 36 and 48.
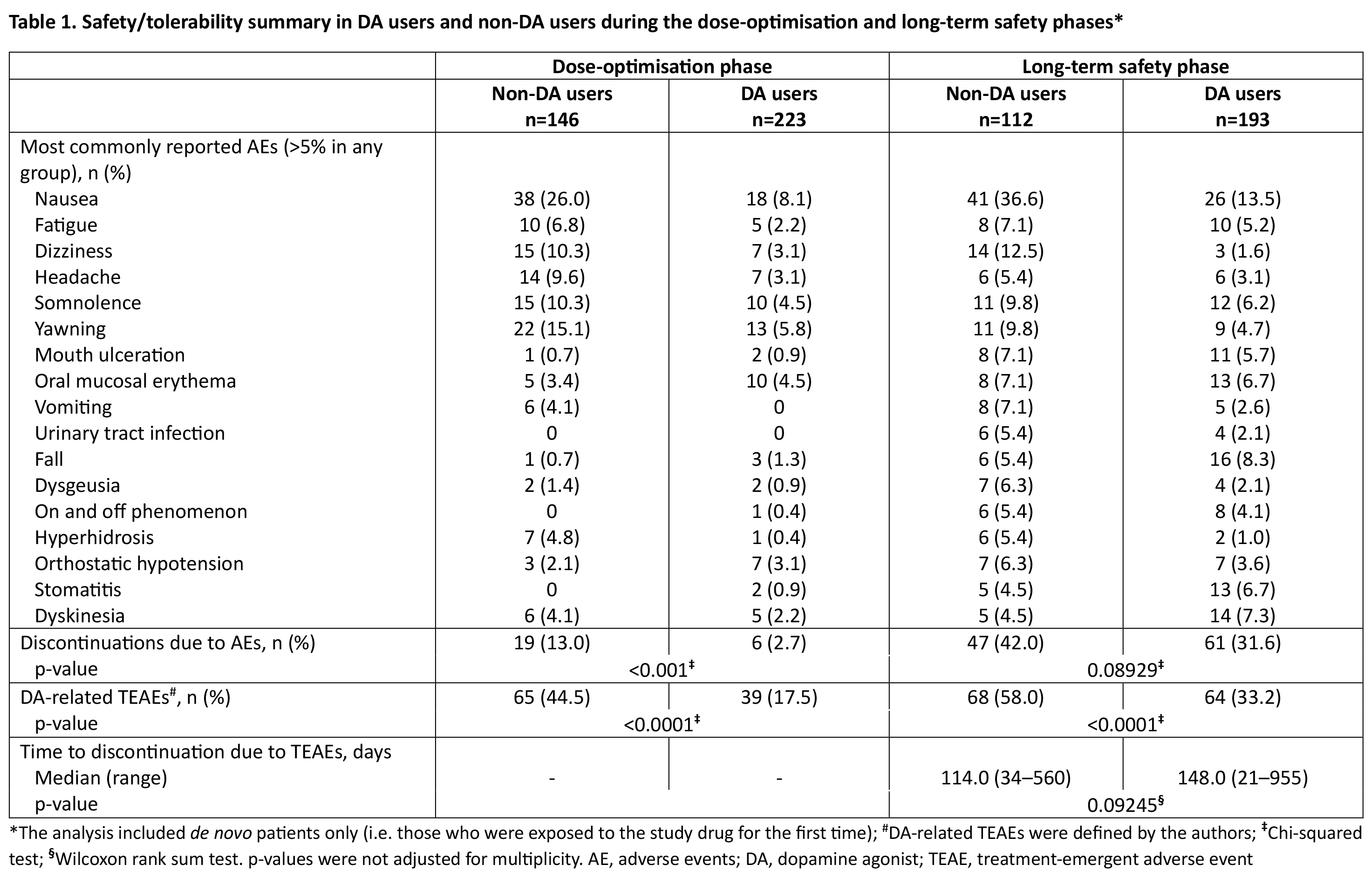
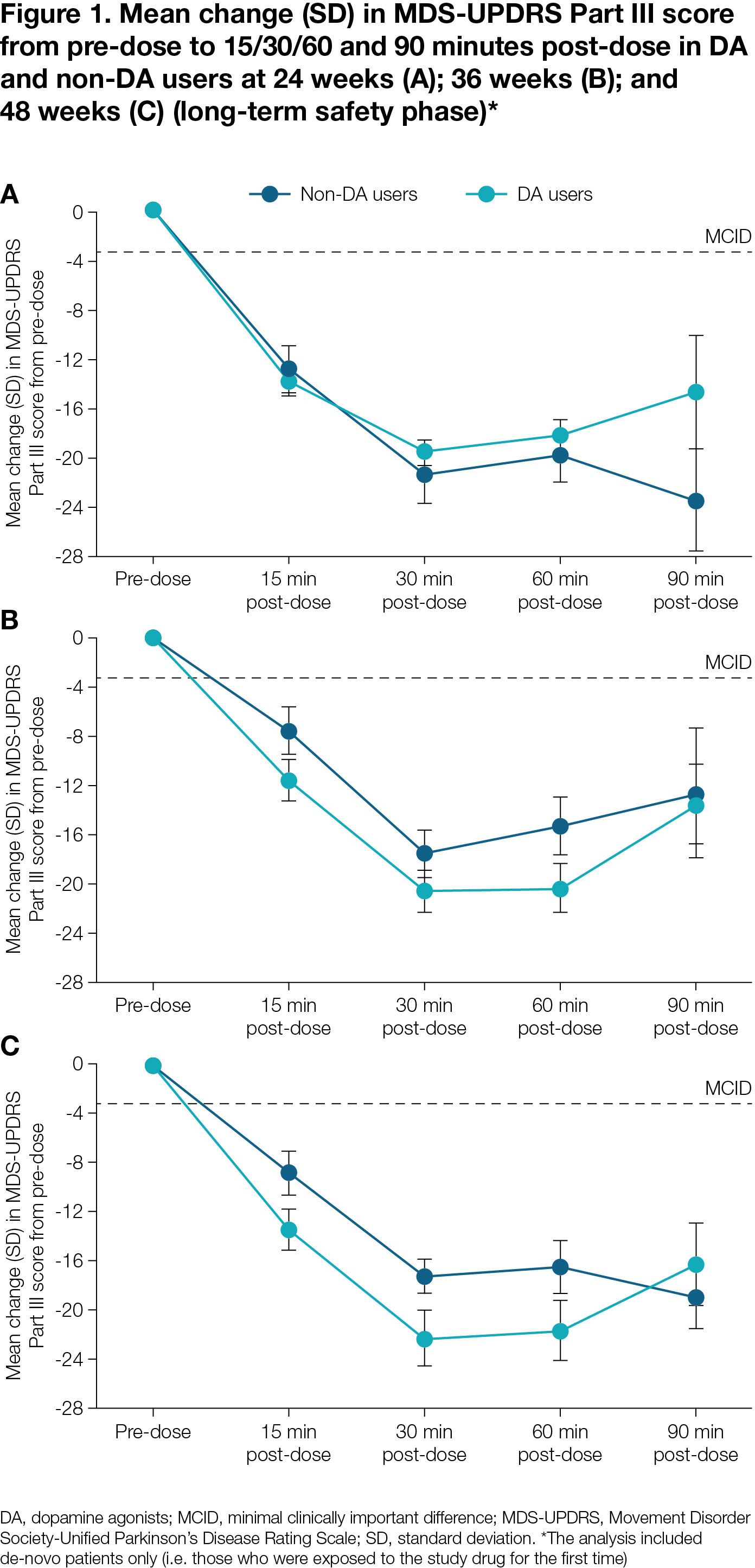
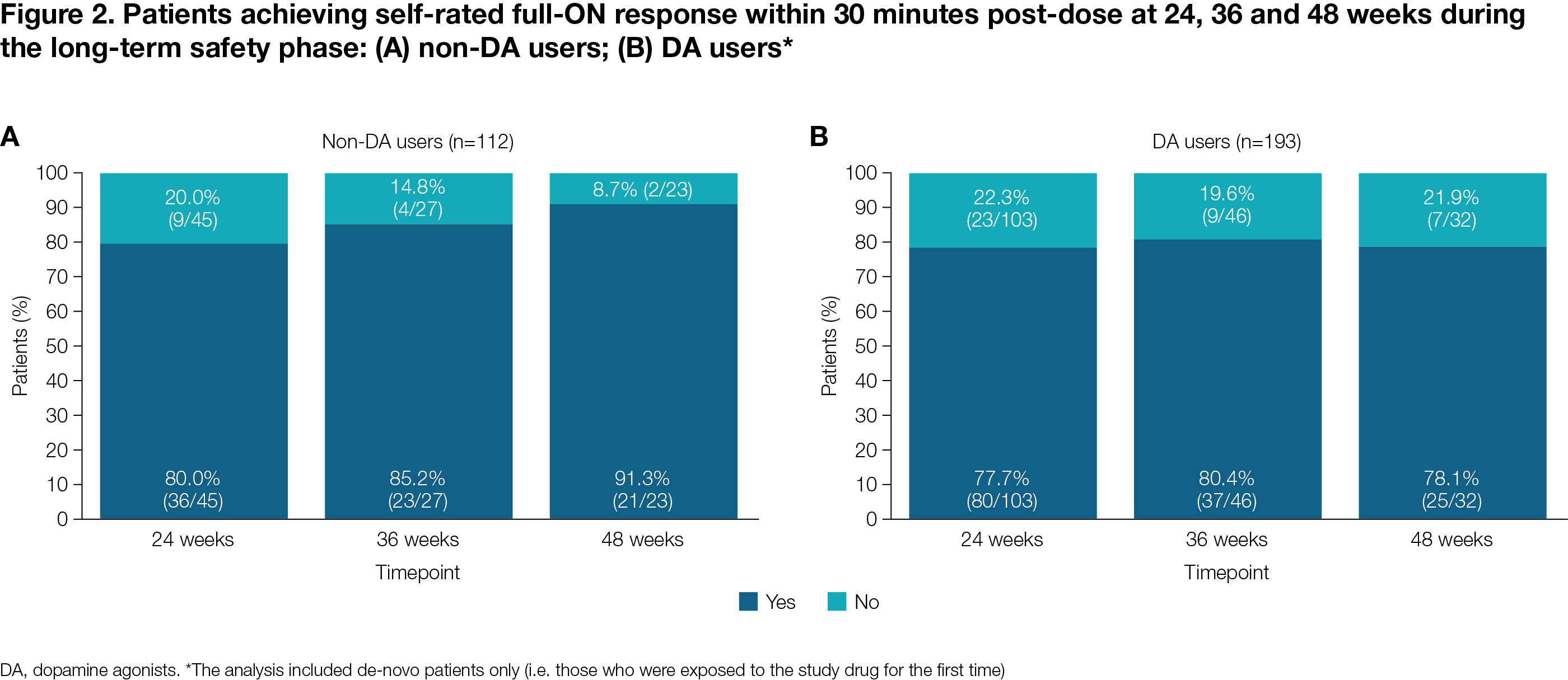
Results: DA vs non-DA users demonstrated lower incidence of most common (>5%) TEAEs, including DA-related TEAEs, and lower discontinuation rates due to TEAEs in both the DO and LTS phases, and remained longer in the study (median 148.0 vs 114.0 days; LTS phase) [Table 1]. Non-DA users had a lower SL-APO mean dose (18.0 vs 21.2 mg) and lower discontinuation rate due to lack of efficacy (4.8 vs 7.2%). For both groups, a clinically meaningful reduction in MDS-UPDRS Part III was reached [Figure 1] and the percentage of patients reporting full-ON response was >75% at all visits [Figure 2].
Conclusion: While SL-APO was found to be better tolerated in DA users, it demonstrated efficacy in both DA and non-DA users.
Table 1
Figure 1
Figure 2
To cite this abstract in AMA style:
D. Santos Garcia, W. Jost, M. José Martí, M. Fonseca, C. Denecke Muhr, I. Pijuan. Safety and Efficacy of Sublingual Apomorphine in Parkinson’s Patients with or without Concomitant Dopamine Agonists Use [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/safety-and-efficacy-of-sublingual-apomorphine-in-parkinsons-patients-with-or-without-concomitant-dopamine-agonists-use/. Accessed December 16, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-and-efficacy-of-sublingual-apomorphine-in-parkinsons-patients-with-or-without-concomitant-dopamine-agonists-use/