Category: Cognitive Disorders (non-PD)
Objective: To evaluate the safety profile of pimavanserin in patients with neuropsychiatric symptoms related to neurodegenerative disease (NDD).
Background: Patients with NDD including Parkinson’s disease (PD) often experience neuropsychiatric symptoms.1,2 These symptoms can greatly impair daily function and lead to reduced quality of life. Treatment options are limited, representing a substantial unmet need. Pimavanserin, a 5-HT2A receptor inverse agonist/antagonist, is FDA-approved to treat PD psychosis.3
Method: This Phase 3b, multicenter, randomized, double-blind, placebo-controlled, parallel-group study evaluated the safety of pimavanserin (34 mg; PIM) versus placebo (PBO) in frail adults and elderly patients with neuropsychiatric symptoms related to NDD. The primary endpoint was safety and tolerability measured by TEAEs. Secondary safety endpoints included change from baseline in motor (Extrapyramidal Symptom Rating Scale-Abbreviated [ESRS-A]) and cognitive function (Mini-Mental State Examination [MMSE]) at Week 8. Exploratory efficacy endpoints included Clinical Global Impression-Severity (CGI-S) and -Improvement (CGI-I), 5-level version of EQ-5D (EQ-5D-5L), and Sleep Disorders Inventory (SDI).
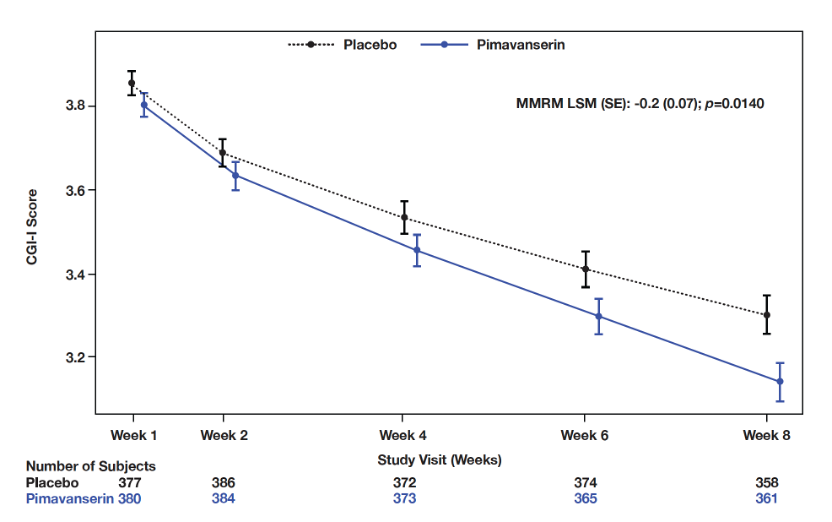
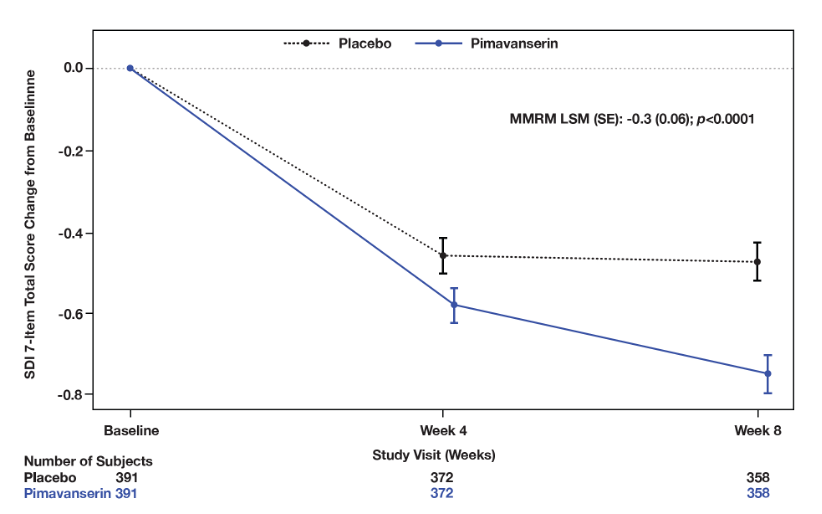
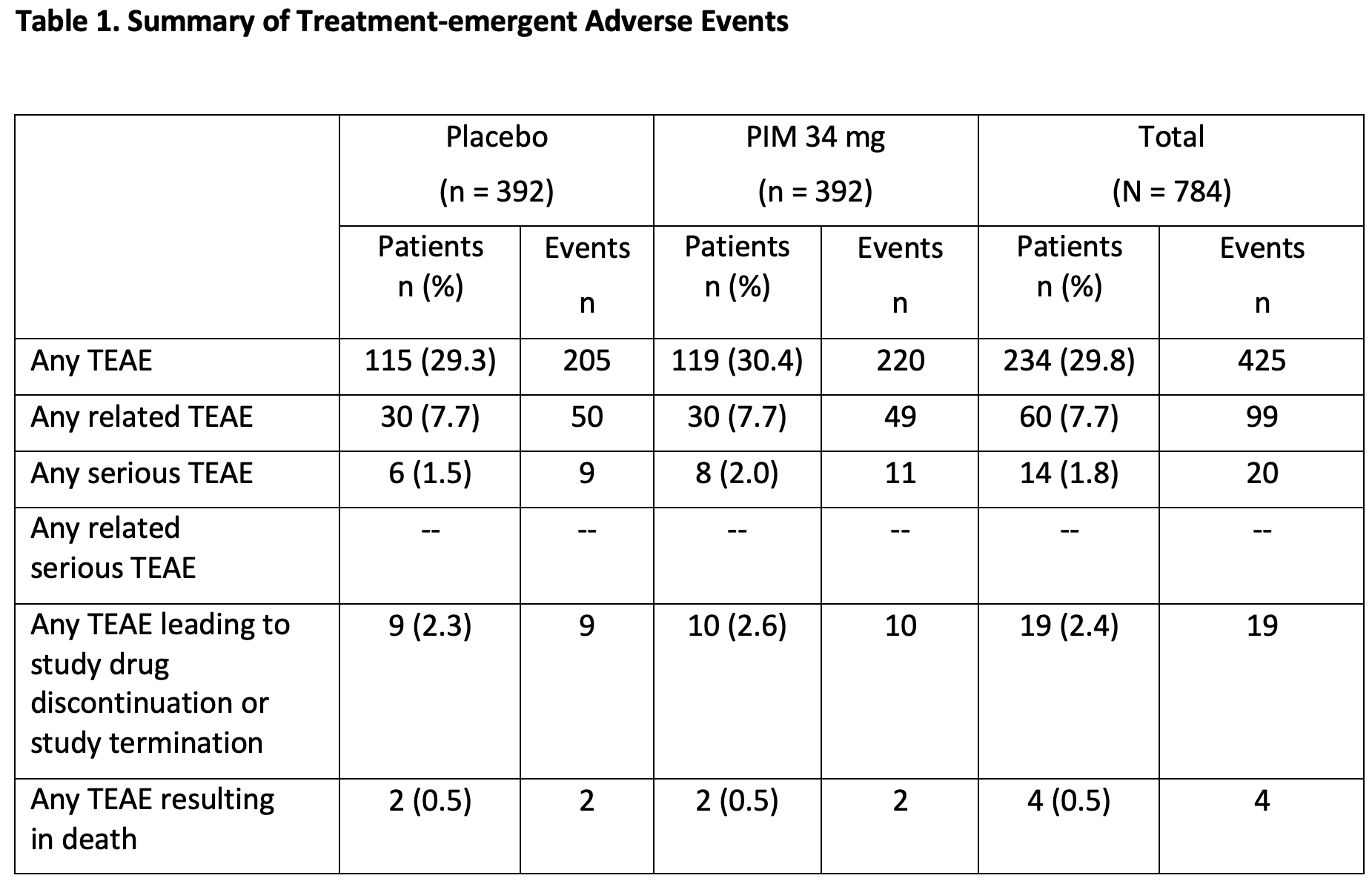
Results: Incidence of TEAEs were similar between PIM (n=392) and PBO (n=392); 29.8% reported ≥1 TEAE (PIM: 30.4%; PBO: 29.3%) and 1.8% reported serious TEAEs (PIM: 2.0%; PBO: 1.5%) (Table 1). TEAEs leading to discontinuation or study termination were reported in 2.6% and 2.3% of patients on PIM and PBO, respectively. The most frequently reported TEAEs were UTI (PIM: 6.4%; PBO: 4.1%) and headache (PIM: 2.0%; PBO: 3.8%). Mortality due to TEAEs was similar between groups (0.5% each). PIM did not impact motor- or cognition-related function (ESRS-A and MMSE); an improvement was seen in CGI-I (MMRM LSM [SE]: -0.2 [0.07], p= 0.0140] and sleep function (SDI) at Week 8 (MMRM LSM [SE]: -0.3 [0.06], p<0.0001] compared to PBO (Figures 1, 2); no differences were observed across other measures.
Conclusion: Pimavanserin was well tolerated in patients with neuropsychiatric symptoms related to NDD, consistent with the known safety profile of pimavanserin, informing its use in PD psychosis. Pimavanserin was not associated with cognitive decline or motor dysfunction.
Previously presented at AAIC July 16-20, 2023
References: 1. Steinberg M, Shao H, Zandi P, et al. Point and 5-year period prevalence of neuropsychiatric symptoms in dementia: the Cache County Study. Int J Geriatr Psychiatry. 2008;23(2):170-177.
2. Lanctôt KL, Amatniek J, Ancoli-Israel S, et al. Neuropsychiatric signs and symptoms of Alzheimer’s disease: New treatment paradigms. Alzheimers Dement (NY). 2017 5;3(3):440-449.
3. Kitten AK, Hallowell SA, Saklad SR, Evoy KE. Pimavanserin: A novel drug approved to treat Parkinson’s Disease Psychosis. Innov Clin Neurosci. 2018;15(1-2):16-22.
To cite this abstract in AMA style:
G. Alva, W. Cubala, A. Berrio, V. Abler, S. Pathak. Safety profile of pimavanserin therapy in elderly patients with neurodegenerative disease-related neuropsychiatric symptoms: a Phase 3b study [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/safety-profile-of-pimavanserin-therapy-in-elderly-patients-with-neurodegenerative-disease-related-neuropsychiatric-symptoms-a-phase-3b-study/. Accessed February 7, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-profile-of-pimavanserin-therapy-in-elderly-patients-with-neurodegenerative-disease-related-neuropsychiatric-symptoms-a-phase-3b-study/