Category: Parkinson’s Disease: Clinical Trials
Objective: Evaluate efficacy and safety outcomes for different patient subgroups categorized according to their baseline characteristics from a Phase 3 study of ND0612.
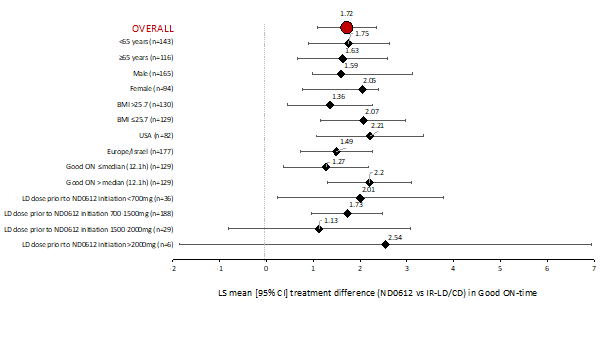
Background: Primary analyses from this Phase 3 study (NCT04006210) showed that treatment with an investigational, subcutaneous infusion of levodopa/carbidopa (ND0612, doses up to 720/90mg/day) supplemented with oral levodopa provided an additional 1.72h [95%CI: 1.08h, 2.36h] of ON-time without troublesome dyskinesia (Good-ON) compared with immediate-release levodopa/carbidopa (IR-LD/CD; p<0.0001) at week 12.
Method: This was a randomized, double-blind, active-controlled trial in patients with Parkinson’s disease experiencing motor fluctuations. Subgroups were analyzed separately for Good-ON using ANCOVA on multiply-imputed data, with additional fixed factors for the subgroup variable and interaction term between the treatment group and subgroup variable. The influence of each subgroup factor was investigated by the interaction terms using Type III p-value combined for multiple imputation.
Results: The adjusted mean [95% CI] treatment effect of ND0612 for Good-ON was homogeneous across the different analyzed subgroups (Figure). Occurrence of adverse events (AEs) and serious AEs were generally consistent across subgroups (age, gender, region, and BMI). Consistent with the data for the full safety population, the most common AEs with ND0612 treatment across all subgroups were infusion site reactions. Overall, no relevant differences between subgroups were observed for AEs of particular interest, including dyskinesia, hallucinations, or falls.
Conclusion: The overall treatment effect was homogenous across different analyzed subgroups. Findings from these analyses support improved Good-ON time, consistent with the overall effect of 1.72h, and no relevant differences in safety or tolerability were observed.
Subgroup analyses of Good ON time
To cite this abstract in AMA style:
W. Poewe, A. Espay, N. Lopes, J. Ferreira. Subgroup Analyses of a Phase 3 Randomized Study of Levodopa/Carbidopa Infusion (ND0612) for Parkinson’s Patients [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/subgroup-analyses-of-a-phase-3-randomized-study-of-levodopa-carbidopa-infusion-nd0612-for-parkinsons-patients/. Accessed February 2, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/subgroup-analyses-of-a-phase-3-randomized-study-of-levodopa-carbidopa-infusion-nd0612-for-parkinsons-patients/