Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To determine the representativity of patients with PSP eligible to clinical trials compared to consecutive PSP patients seen in a reference center and the time window for eligibility.
Background: Progressive supranuclear palsy (PSP) is a parkinsonian syndrome with no cure. Recently, the MDS published revised diagnosis criteria to provide early and reliable diagnosis of PSP and its variants. Two large randomized clinical trials were initiated in 2017.
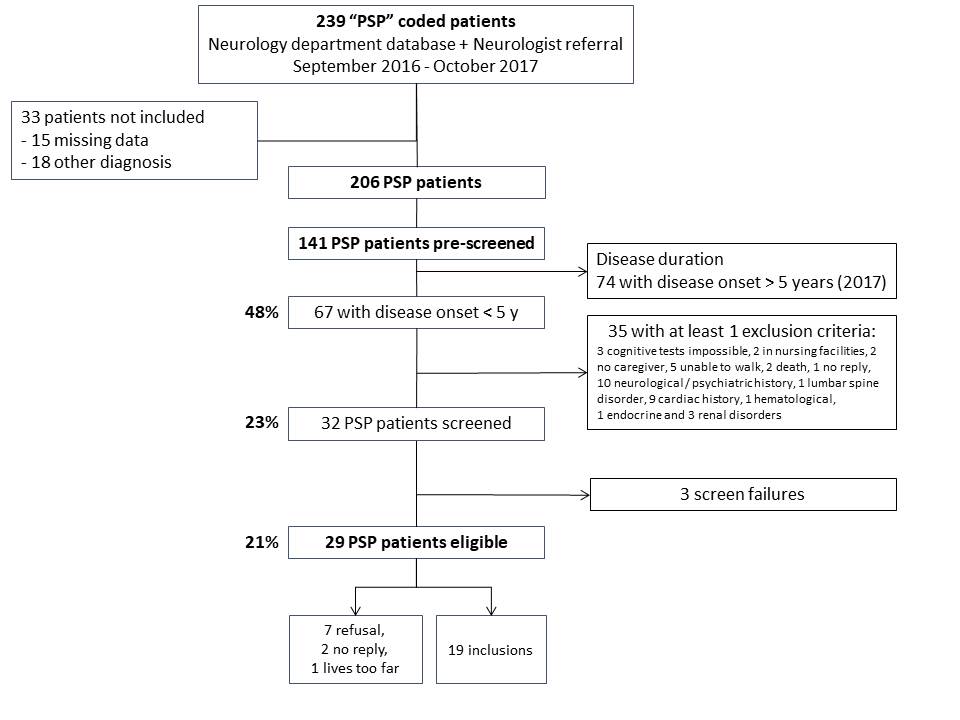
Method: We conducted a single center retrospective study of PSP patients referred to a tertiary department of Neurology (Pitié-Salpêtrière Hospital, Paris) for clinical diagnosis and clinical trial inclusion, over a 12-month period. We assessed the relative proportions of different PSP subtypes, as defined by the MDS-PSP criteria, in the whole population compared to patients eligible in trials
Results: 206 PSP patients were included, among which 175 (85%) were diagnosed with probable PSP-Richardson’s syndrome (RS) subtype, with a mean age of 73 and a mean disease duration of 5 years (See figure 1). Among those patients, 29 (21%) were eligible (age 71±10.7, disease duration 3.1±1.2 years) and 19 were included in trials, all with a diagnosis of probable PSP-RS. As compared to the whole population, patients included in clinical trials tended to be younger, and showed more PSP-RS subtypes (p<0.05)
Conclusion: The PSP population included in trials is very similar to the general PSP population, but younger, with shorter disease duration. By definition, only probable PSP subtypes are included in clinical trials. The time window for inclusion is short because of diagnosis delay, fast disease progression and old age of the population.
To cite this abstract in AMA style:
LL. Mariani, R. Guimarães-Costa, D. Grabli, B. Letoullec, F. Cormier, B. Degos, B. Dubois, M. Vidailhet, L. Lacomblez, JC. Corvol. The Representativity of PSP patients included in clinical trials [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/the-representativity-of-psp-patients-included-in-clinical-trials/. Accessed February 27, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/the-representativity-of-psp-patients-included-in-clinical-trials/