Session Information
Date: Thursday, June 8, 2017
Session Title: Parkinson’s Disease: Clinical Trials, Pharmacology And Treatment
Session Time: 1:15pm-2:45pm
Location: Exhibit Hall C
Objective: To use clinical and biological data from PPMI to estimate sample size for disease modification studies in de novo PD
Background: One barrier to using biological markers as clinical trial outcomes is lack of longitudinal data on the rate of change
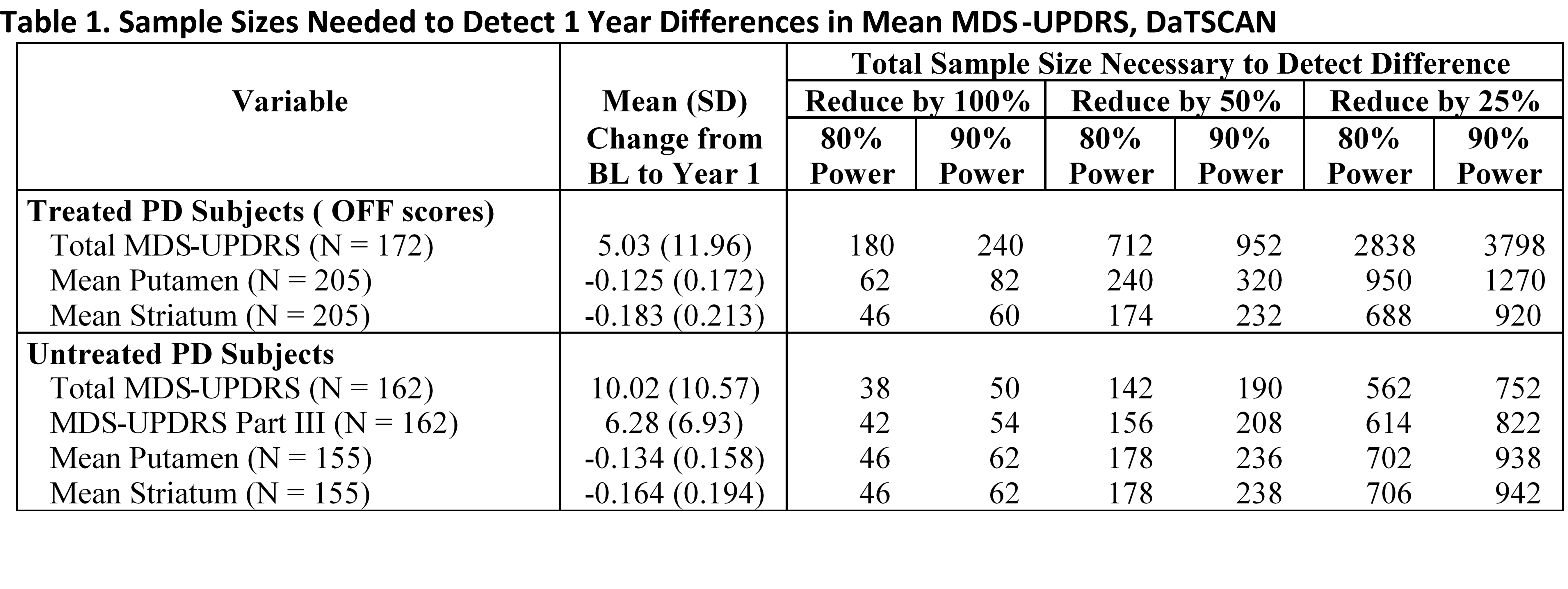
Methods: PPMI PD participants and healthy controls are assessed annually with a spectrum of motor and non-motor scales, DAT imaging and biologic variables. For participants on dopaminergic therapy (DT), MDS-UPDRS motor scale is assessed in the defined OFF (>6 hours post last DT) and ON states. We used change in MDS- UPDRS, DAT imaging and CSF measures (α-synuclein, tau, a-beta) to model sample size.
Results: MDS-UPDRS I-III and mean putamen and striatum DAT scores allow the smallest sample size. CSF variables would require a substantially larger sample size. Extending the follow up to 2 years would reduce the sample size by nearly 50%.
[table1]
Conclusions: Recruiting PD patients who can remain untreated for 12 months is most efficient but will not represent the entire PD population. Using DAT imaging as an outcome substantially reduces sample size in DAT treated PD patients. A two year study duration reduces sample size but must be balanced with retention challenges and overall cost.
References: Authorship: Ken Marek for the PPMI Investigators
To cite this abstract in AMA style:
T. Simuni, C. Caspell-Garcia, N. Seedorff, C. Coffey, S. Lasch, B. Mollenhauer, C. Tanner, K. Kieburtz, K. Marek. Sample size estimation for clinical trials in de novo Parkinson’s disease (PD): Results from the Parkinson’s Progression Markers Initiative (PPMI) Study [abstract]. Mov Disord. 2017; 32 (suppl 2). https://www.mdsabstracts.org/abstract/sample-size-estimation-for-clinical-trials-in-de-novo-parkinsons-disease-pd-results-from-the-parkinsons-progression-markers-initiative-ppmi-study/. Accessed February 2, 2026.« Back to 2017 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/sample-size-estimation-for-clinical-trials-in-de-novo-parkinsons-disease-pd-results-from-the-parkinsons-progression-markers-initiative-ppmi-study/