Session Information
Date: Wednesday, June 22, 2016
Session Title: Neuroimaging (non-PD)
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To evaluate: (1) brain tau pathology in vivo in patients with Progressive Supranuclear Palsy (PSP) and Alzheimer’s disease (AD); (2) whether brain tau pathology (indexed by 18FAV-1451 PET) correlates with disease severity, (3) the feasibility of using [18F]AV-1451 as an in vivo tau biomarker to discriminate between patients with PSP and AD.
Background: In studies of rodent models and ex-vivo AD human brains, the radiotracer [18F]AV-1451 co-localizes with hyper-phosphorylated tau. Here we test whether [18F] AV-1451 reliably differentiates PSP and AD and if it matches the post mortem distribution of tau pathology.
Methods: [18F]AV-1451 PET was used to compare the patterns of tau pathology in n=13 PSP patients, n=9 AD patients, n=6 MCI with positive PiB scan, and 8 controls
| PSP (n=13) | AD/MCI+ (n=15) | HCs (n=8) | Group differences | |
| Sex (males/females) | 7/6 | 9/6 | 3/5 | ns |
| Age, yrs. (mean ± SD) | 69.8 (±6.4) | 70.8 (±8.7) | 64.9 (±8.9) | F=1.3, p=0.25 |
| Education, yrs. (mean ± SD) | 11.8 (±1.4) | 14.3 (±3.3) | 16.0 (±1.6) | F=7.2, p=0.002 |
| MMSE scores (mean ± SD) | 25.1 (±5.1) | 25.5 (±2.8) | 29.8 (±0.4) | F=4.4, p=0.018 |
| ACE-R scores (mean ± SD) | 74.5 (±17.1) | 75.9 (±11.0) | 95.9 (±3.1) | F=7.7, p=0.002 |
Results: Relative to controls, both PSP and AD groups showed elevated [18F] AV-1451 binding, but in very different locations 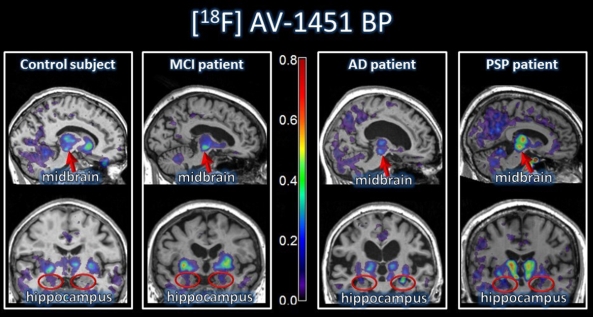
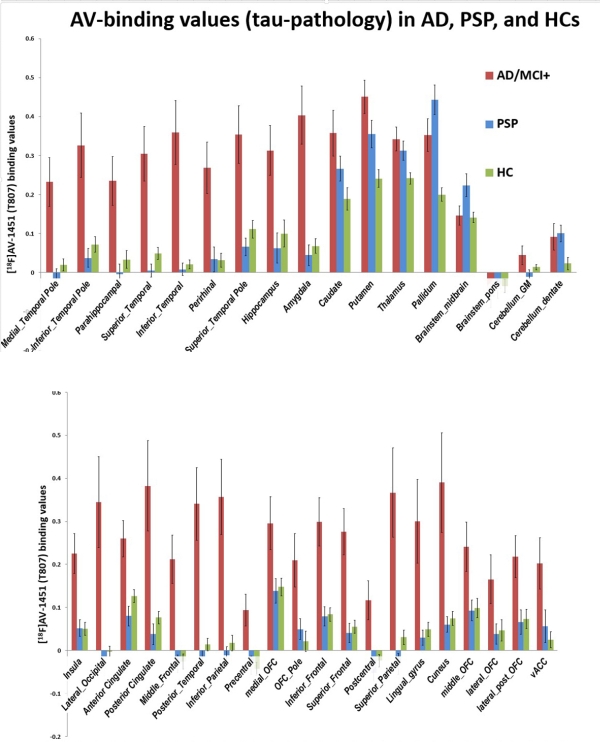 . PSP patients displayed increased [18F]AV-1451 uptake in the midbrain and pallidum, consistent with post mortem studies. AD patients showed increased [18F]AV-1451 uptake in the hippocampus, amygdala, and other medial temporal lobe areas as well as extensive cortical regions. No significant correlations between [18F]AV-1451 uptake in any ROI and clinical and neuropsychological measures of disease severity were identified. SVM analyses showed that the binding of [18F]AV-1451 in the midbrain and hippocampus separated PSP from AD groups with an accuracy of 96%.
. PSP patients displayed increased [18F]AV-1451 uptake in the midbrain and pallidum, consistent with post mortem studies. AD patients showed increased [18F]AV-1451 uptake in the hippocampus, amygdala, and other medial temporal lobe areas as well as extensive cortical regions. No significant correlations between [18F]AV-1451 uptake in any ROI and clinical and neuropsychological measures of disease severity were identified. SVM analyses showed that the binding of [18F]AV-1451 in the midbrain and hippocampus separated PSP from AD groups with an accuracy of 96%.
Conclusions: Our results suggest that [18F]AV-1451 PET is a useful in vivo tool to assess brain tau pathology in PSP and AD. [18F]AV-1451 may improve the diagnostic protocols in PSP and AD and be a useful outcome measure in trials that aim to halt the tau-related neurodegenerative disorders.
To cite this abstract in AMA style:
J.B. Rowe, P. Vázquez Rodríguez, Y.T. Hong, R.J. Borchert, S. Sami, W.R. Bevan-Jones, S.P. Jones, R. Arnold, A. Surendranathan, E. Mak, S. Li, T. Fryer, J. O'Brien, L. Passamonti. [18F]AV-1451 PET imaging of tau in progressive supranuclear palsy and Alzheimer’s disease [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/18fav-1451-pet-imaging-of-tau-in-progressive-supranuclear-palsy-and-alzheimers-disease/. Accessed February 11, 2026.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/18fav-1451-pet-imaging-of-tau-in-progressive-supranuclear-palsy-and-alzheimers-disease/
