Objective: The phase 2b KINETIC 2 trial is a double-blind, randomized, placebo-controlled, dose-response study designed to evaluate SAGE-324/BIIB124 (SAGE-324) for the treatment of patients with essential tremor (ET; NCT05173012).
Background: ET is a common movement disorder that has been associated with altered gamma-aminobutyric acid (GABA) neurotransmission. Current medical treatment options for ET may not provide optimal tremor control for some patients [1]. SAGE-324 is an investigational GABA type A receptor–positive allosteric modulator that was evaluated in the proof-of-concept phase 2 KINETIC trial. The primary endpoint of KINETIC was met: patients with ET receiving SAGE-324 once daily experienced a statistically significant reduction in upper limb tremor as assessed by change from baseline (CFB) in The Essential Tremor Rating Assessment Scale-Performance Subscale [TETRAS-PS] Item 4 at Day 29 compared with those receiving placebo; 33/34 (97.1%) patients in the SAGE-324 group had a treatment-emergent adverse event of any grade.
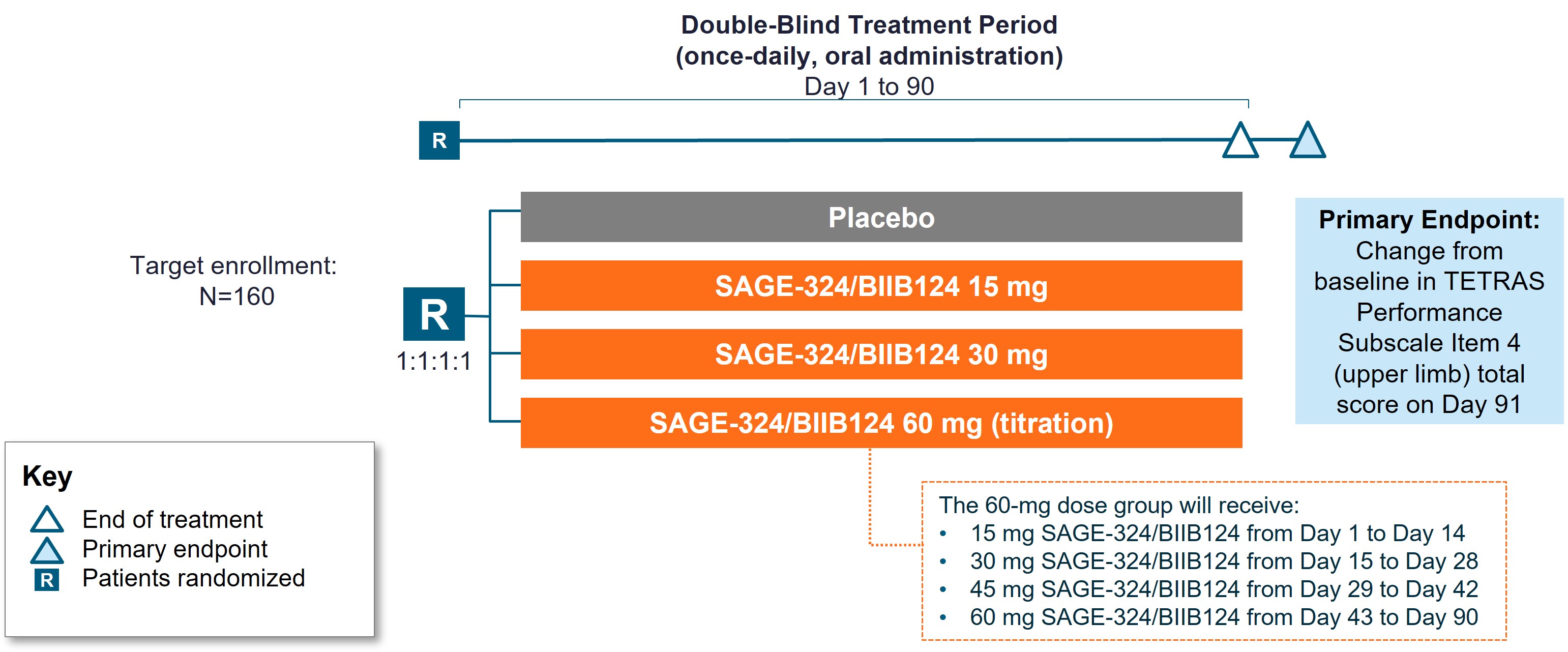
Method: The KINETIC 2 trial is planned to enroll 160 patients from up to 50 sites in the United States. Key eligibility criteria include: age 18-80 years; ET diagnosis (isolated tremor syndrome consisting of bilateral upper limb action tremor for ≥3 years with or without tremor in other locations); no other relevant neurological signs, a TETRAS-PS Item 4 score of ≥12 at screening and at baseline with a total score of ≥6 for the dominant upper limb, and a baseline TETRAS Activities of Daily Living (ADL) score of ≥20 at screening; and willingness to discontinue medications taken to treat ET prior to receiving SAGE-324.
Patients are randomized 1:1:1:1 to 1 of 4 blinded treatment groups (placebo or SAGE-324: 15 mg, 30 mg, or 60 mg oral, daily at night). [figure1]. The SAGE-324 60-mg dose will be reached after blinded uptitration from 15 mg over 42 days.
The primary endpoint is CFB in TETRAS-PS Item 4 score on Day 91, and the secondary endpoint is CFB in TETRAS ADL composite score. Safety and tolerability of SAGE-324 will be assessed.
Results: The ongoing KINETIC 2 trial is estimated to complete in 2023.
Conclusion: The phase 2b KINETIC 2 trial was designed to evaluate SAGE-324 dose response on clinically relevant endpoints, including ADL, and safety. Enrollment is ongoing, and the results of this trial will inform the clinical development of SAGE-324.
References: [1] Hedera P. Ther Adv Neurol Disord. 2017;10(2):137-148.
To cite this abstract in AMA style:
R. Pahwa, A. Ellenbogen, R. Elble, K. Bankole, T. Dam, J. Fratantonio, J. Gaiha-Rohrbach, M. Qin, S. Garafola, M. Gerbasi, H. Colquhoun. A phase 2b, randomized, dose-response study of SAGE-324/BIIB124 for the treatment of essential tremor: KINETIC 2 trial in progress [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/a-phase-2b-randomized-dose-response-study-of-sage-324-biib124-for-the-treatment-of-essential-tremor-kinetic-2-trial-in-progress/. Accessed March 4, 2026.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-phase-2b-randomized-dose-response-study-of-sage-324-biib124-for-the-treatment-of-essential-tremor-kinetic-2-trial-in-progress/