Session Information
Date: Monday, September 23, 2019
Session Title: Clinical Trials, Pharmacology and Treatment
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: Evaluate if patients fulfill the recently developed “5-2-1” screening criteria for advanced Parkinson’s Disease (APD)[1] prior to treatment with levodopa-carbidopa intestinal gel (LCIG) and to assess effectiveness outcomes during routine care in respective subgroups.
Background: A Delphi expert consensus panel proposed that fulfilling at least one of the following criteria would suggest possible APD: ≥5 times oral levodopa taken/day, ≥2 hours of OFF time/day, or ≥1 hour/day of troublesome dyskinesia (TSD)[1].
Method: DUOGLOBE is a global, multicenter, single-arm, postmarketing observational study assessing the long-term effectiveness of LCIG in APD patients. In this post-hoc analysis of the 1st interim dataset, patients were stratified into subgroups who met all, one or more, and each individual 5‑2‑1 criteria at baseline. Assessments included the Unified Dyskinesia Rating Scale (UDysRS), Non-motor Symptom Scale (NMSS), and Parkinson’s Disease Questionnaire (PDQ-8) from baseline to 6 months of follow-up.
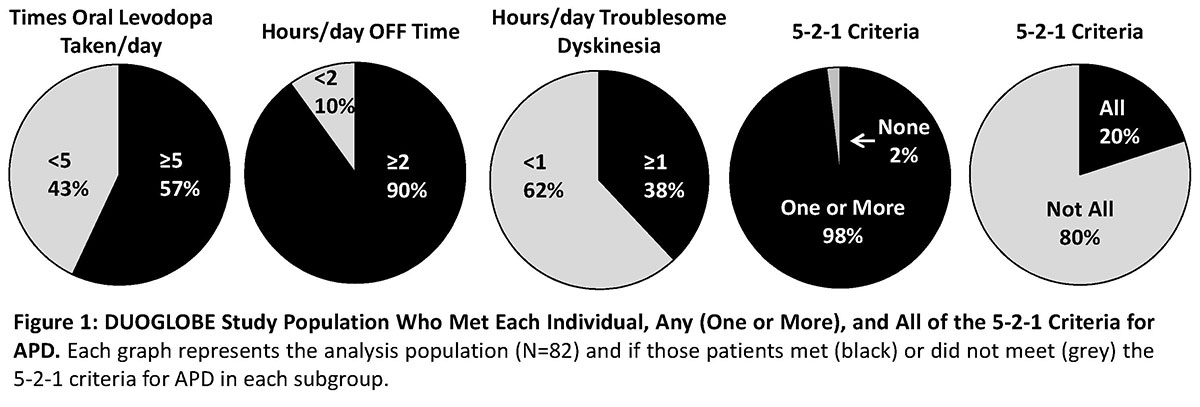
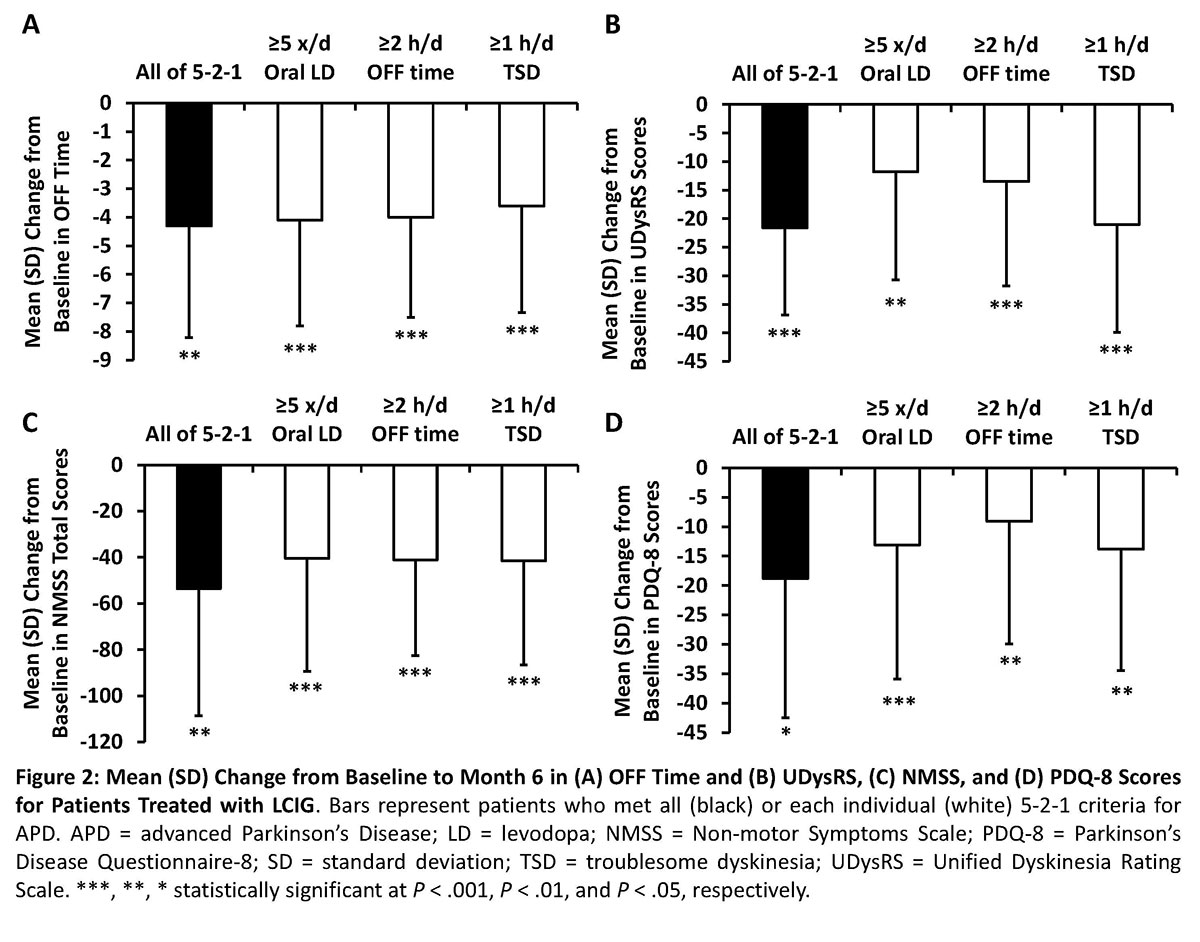
Results: At baseline, 80 patients (98%) met ≥1 of the 5-2-1 criteria and 16 patients (20%) met all criteria (Figure 1). More patients at baseline fulfilled the OFF time criterion (90%) than the ≥5 times oral levodopa taken/day (57%) or ≥1 hour TSD/day (38%)(Figure 1). Patients that met none of the 5-2-1 criteria or had <2 hours of OFF time had insufficient sample sizes for analysis (both, n<8). All analyzed subgroups had significant (P < .01) improvements in OFF time and UDysRS scores from baseline to month 6 with LCIG treatment (Figure 2). Patients who met ≥1 of the 5-2-1 criteria had significant (P < .05) improvements in NMSS total and PDQ‑8 scores (Figure 2). Across all subgroups, 6%-16% patients discontinued the study; the main reasons for discontinuation were adverse events (0%-11%), withdrawn consent (0%-9%), and other (3%-7%).[figure1][figure2]
Conclusion: This interim analysis demonstrates that almost all patients selected for LCIG by DUOGLOBE investigators on clinical grounds fulfilled ≥1 of the 5-2-1 criteria, suggesting usefulness of 5‑2‑1 as a screening tool for APD when considering device-aided therapies. Patients treated with LCIG who met ≥1 of the 5-2-1 criteria had improvements in OFF time, dyskinesia, NMS, and QOL, and these data support the established safety profile of LCIG treatment in phase 3 trials.
References: 1. Antonini A, Stoessl AJ, Kleinman LS, et al. Developing consensus among movement disorder specialists on clinical indicators for identification and management of advanced Parkinson’s disease: a multi-country Delphi-panel approach. Curr Med Res Opin. 2018. Dec;34(12):2063-2073.
To cite this abstract in AMA style:
A. Antonini, J. Aldred, L. Bergmann, N. Kovács, P. Kukreja, W. Robieson, D. Standaert, KR. Chaudhuri. Application of the “5-2-1” Screening Criteria in Advanced Parkinson’s Disease Patients Treated with Levodopa-Carbidopa Intestinal Gel: Interim Analysis from the DUOGLOBE Study [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/application-of-the-5-2-1-screening-criteria-in-advanced-parkinsons-disease-patients-treated-with-levodopa-carbidopa-intestinal-gel-interim-analysis-from-the-duoglobe-study/. Accessed February 21, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/application-of-the-5-2-1-screening-criteria-in-advanced-parkinsons-disease-patients-treated-with-levodopa-carbidopa-intestinal-gel-interim-analysis-from-the-duoglobe-study/