Session Information
Date: Monday, September 23, 2019
Session Title: Huntington’s Disease
Session Time: 1:45pm-3:15pm
Location: Agora 3 West, Level 3
Objective: To explore the effect of laquinimod on brain volume in patients with Huntington Disease (HD).
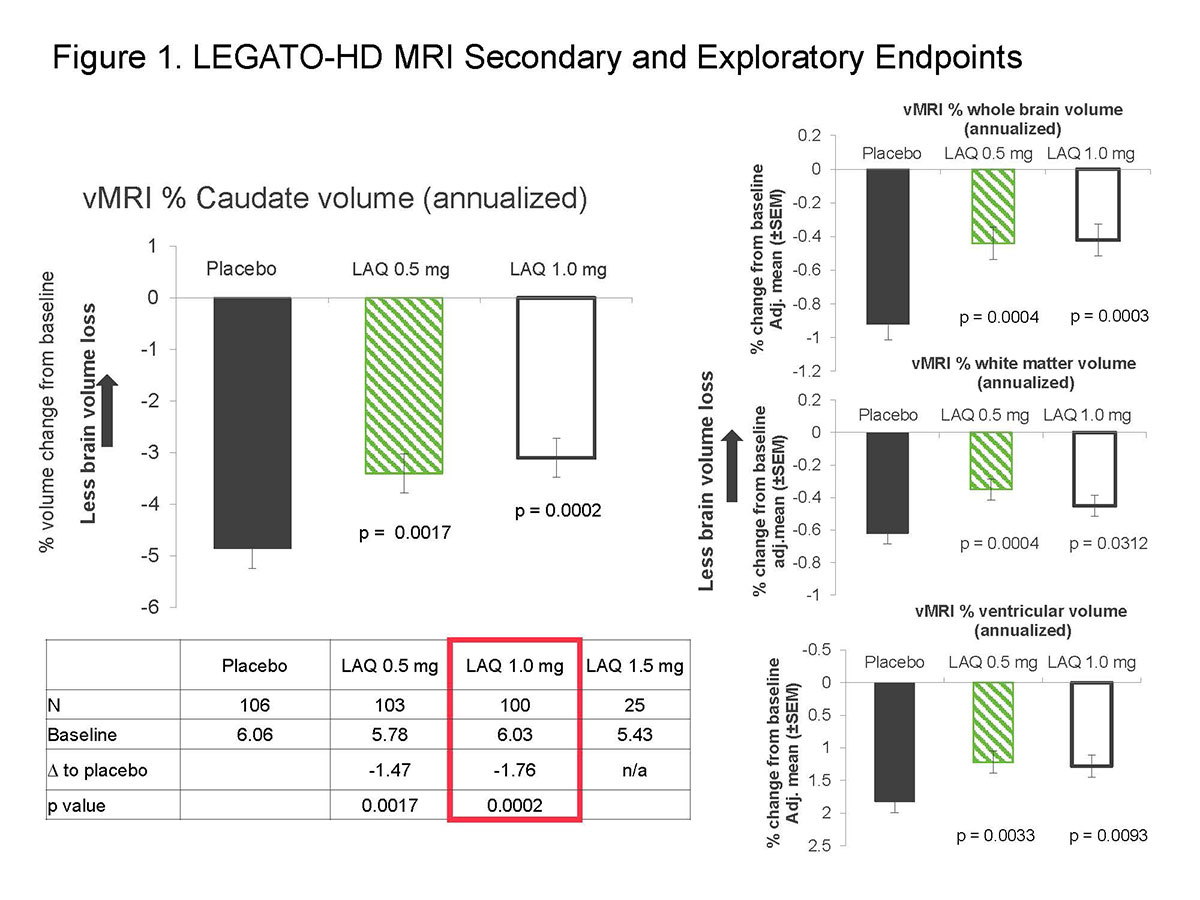
Background: Volume loss in caudate and other brain regions is a hallmark of HD pathology and has been shown to correlate with motor and clinical outcomes in HD studies. Laquinimod shows improvement in HD animal models including rescue of striatal and callosal atrophy. In the phase 2 LEGATO-HD study, the primary endpoint of change from baseline at week 52 of treatment in UHDRS-TMS scores was not met. The present report describes results for its secondary endpoint (EP) of percent change from baseline in caudate volume (CV), MRI exploratory EPs and subgroup analyses (all preplanned).
Method: MRI scans were performed at baseline and week 52 visits. The secondary EP measure was percent CV change (1.0 mg once daily) and the exploratory EP measures were percent CV change (0.5 mg once daily) and percent whole brain volume change, percent white matter volume change, and absolute change in ventricular volume for the 0.5 and 1.0 mg treatment arms. Both the primary EP (TMS) and secondary EP (CV change) were under Type I error control; exploratory EPs were not under Type 1 error control.
Results: For the secondary EP, patients treated with laquinimod 1.0 mg showed reduced CV loss from baseline to week 52 compared to placebo-treated patients (p = 0.0002, Figure 1). All exploratory volumetric data showed consistent treatment effects on brain volume loss at week 52 for laquinimod 0.5 and 1.0 mg doses and across all preplanned subgroups (all p < 0.01).
Conclusion: In LEGATO-HD we identified a consistent lessening of volume loss in caudate and other brain structures in both laquinimod treatment arms compared to placebo, and across all preplanned subgroups, however, this lessening of brain volume loss in the laquinimod group did not correlate with TMS and other clinical outcomes.
To cite this abstract in AMA style:
R. Reilmann, M. Gordon, A. Feigin, K. Anderson, S. Tabrizi, B. Leavitt, J. Stout, P. Piccini, N. Hobbs, R. Manber, B. Borowsky, G. Rynkowski, R. Volkinshtein, J. Savola, M. Hayden. Brain MRI Volume Changes after 12 months laquinimod treatment of Huntington disease (LEGATO-HD) [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/brain-mri-volume-changes-after-12-months-laquinimod-treatment-of-huntington-disease-legato-hd/. Accessed February 6, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/brain-mri-volume-changes-after-12-months-laquinimod-treatment-of-huntington-disease-legato-hd/