Category: Tremor
Objective: To investigate cortical integration of proprioceptive information in patients with orthostatic tremor (OT), measured as event-related synchronisation (ERS) response to proprioceptive stimulation using magnetencephalography (MEG) [1-3].
Background: OT is a rare neurological disorder where a fast synchronised tremor occurs in leg and trunk muscles when activated in standing position [4-6]. Besides of the tremor, patients are strongly affected by an intense feeling of unsteadiness while standing [6-8]. This may be explained by a pathological integration of proprioceptive information either by disturbance of the fast tremor activity affecting afferent proprioceptive signals or by pathological changes impeding the integration of proprioceptive information [9-12].
Method: Fifteen OT patients and fifteen age and gender matched healthy controls (HC) were examined in a whole-head MEG system containing 102 triplets of magnetometer and 2 planar gradiometers. Proprioceptive stimulation by passive movements of the right index finger and the right forefoot was evoked by custom-made pneumatic MEG-compatible actuators [figure 1 and 2] [13,14]. Sensor-level time-frequency responses were extracted in a frequency range of 8 to 30 Hz, including both alpha (8-13 Hz) and beta (14-30 Hz) rhythms. The gradiometer pair with the highest amplitude change time-locked to the proprioceptive stimulus was used to calculate the ERS. Electromyography (EMG) tracked muscle activity on the right arm and leg to enable detection of possible voluntary movements during the passive movements.
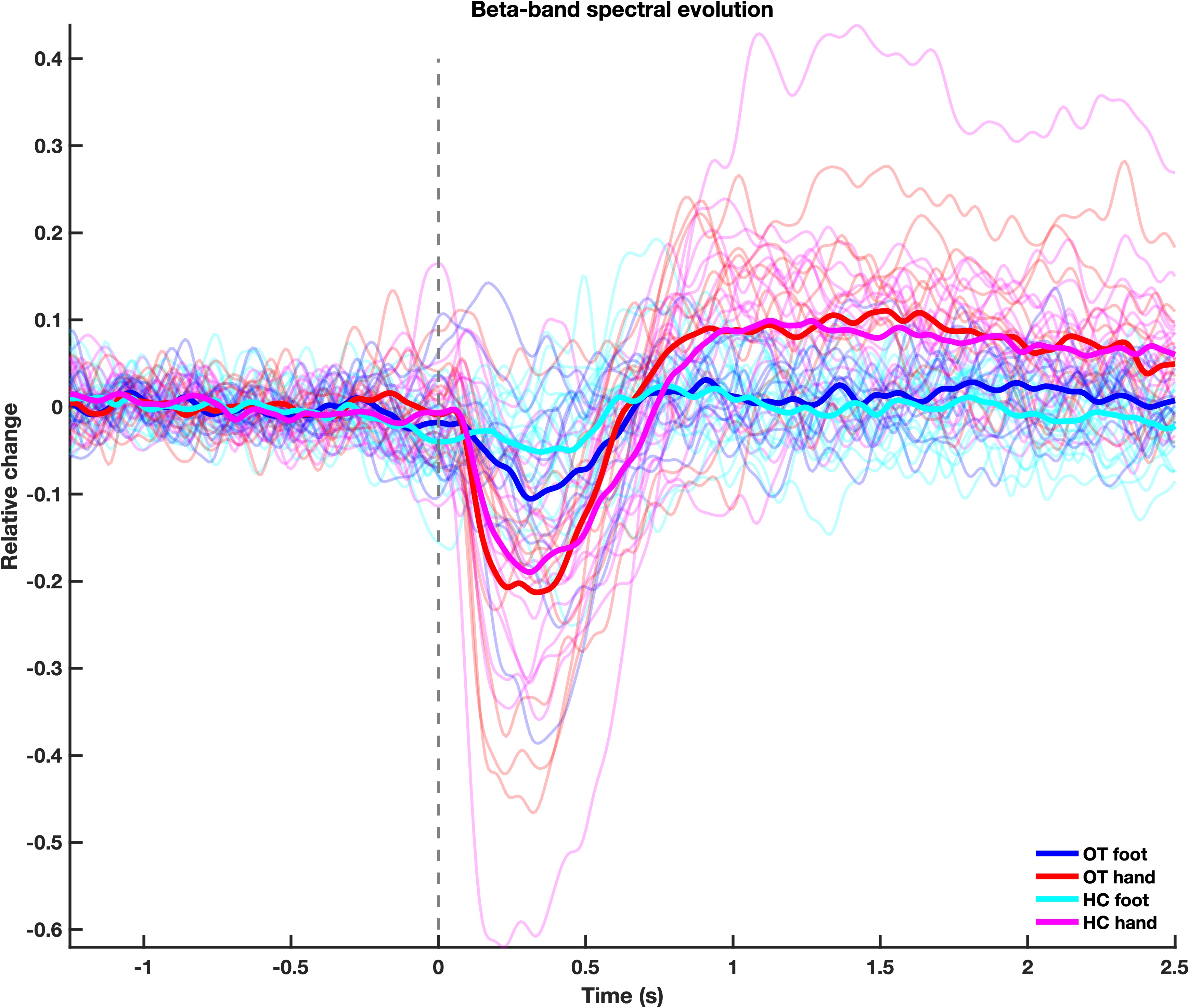
Results: Finger stimulation did induce ERS followed by a rebound as expected in both groups. The stimulation of the foot, however, only induced a slight ERS. Cluster-based permutation tests comparing the HC and OT groups did not result in any significant differences in the time-frequency responses to the proprioceptive stimulation of the right index finger, nor to the right foot [figure 3].
Conclusion: Proprioceptive stimulation induced a stable ERS in OT without evidence for a difference compared with healthy individuals. Thus, the characteristic intense feeling of instability in OT could not be referred to an altered cortical integration of proprioceptive information.
Fig1. Passive movement of the right foot.
Fig2. Passive movement of the right index finger.
Fig3. Time-frequency response.
References: 1. Parkkonen E, Laaksonen K, Piitulainen H, Parkkonen L, Forss N. Modulation of the reverse similar20-Hz motor-cortex rhythm to passive movement and tactile stimulation. Brain Behav. 2015;5(5):e00328.
2. Cheyne DO. MEG studies of sensorimotor rhythms: a review. Exp Neurol. 2013;245:27-39.
3. Piitulainen H, Bourguignon M, De Tiege X, Hari R, Jousmaki V. Corticokinematic coherence during active and passive finger movements. Neuroscience. 2013;238:361-70.
4. Bhatia KP, Bain P, Bajaj N, Elble RJ, Hallett M, Louis ED, et al. Consensus Statement on the classification of tremors. from the task force on tremor of the International Parkinson and Movement Disorder Society. Mov Disord. 2018;33(1):75-87.
5. Piboolnurak P, Yu QP, Pullman SL. Clinical and neurophysiologic spectrum of orthostatic tremor: case series of 26 subjects. Mov Disord. 2005;20(11):1455-61.
6. Hassan A, Ahlskog JE, Matsumoto JY, Milber JM, Bower JH, Wilkinson JR. Orthostatic tremor: Clinical, electrophysiologic, and treatment findings in 184 patients. Neurology. 2016;86(5):458-64.
7. Ganos C, Maugest L, Apartis E, Gasca-Salas C, Caceres-Redondo MT, Erro R, et al. The long-term outcome of orthostatic tremor. J Neurol Neurosurg Psychiatry. 2016;87(2):167-72.
8. Feil K, Bottcher N, Guri F, Krafczyk S, Schoberl F, Zwergal A, Strupp M. Long-term course of orthostatic tremor in serial posturographic measurement. Parkinsonism Relat Disord. 2015;21(8):905-10.
9. Bacsi AM, Fung VS, Colebatch JG. Sway patterns in orthostatic tremor: impairment of postural control mechanisms. Mov Disord. 2005;20(11):1469-75.
10. Fung VS, Sauner D, Day BL. A dissociation between subjective and objective unsteadiness in primary orthostatic tremor. Brain. 2001;124(Pt 2):322-30.
11. Wuehr M, Schlick C, Mohwald K, Schniepp R. Proprioceptive muscle tendon stimulation reduces symptoms in primary orthostatic tremor. J Neurol. 2018;265(7):1666-70.
12. Mohwald K, Wuehr M, Schenkel F, Feil K, Strupp M, Schniepp R. The gait disorder in primary orthostatic tremor. J Neurol. 2020;267(Suppl 1):285-91.
13. Vinding MC, Tsitsi P, Piitulainen H, Waldthaler J, Jousmaki V, Ingvar M, et al. Attenuated beta rebound to proprioceptive afferent feedback in Parkinson’s disease. Sci Rep. 2019;9(1):2604.
14. Piitulainen H, Bourguignon M, Hari R, Jousmaki V. MEG-compatible pneumatic stimulator to elicit passive finger and toe movements. Neuroimage. 2015;112:310-7.
To cite this abstract in AMA style:
K. Af Edholm, M. Vinding, C. Pfeiffer, D. Lundqvist, J. Waldthaler. Cortical response to proprioceptive stimulation in orthostatic tremor – a magnetoencephalography study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/cortical-response-to-proprioceptive-stimulation-in-orthostatic-tremor-a-magnetoencephalography-study/. Accessed July 15, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/cortical-response-to-proprioceptive-stimulation-in-orthostatic-tremor-a-magnetoencephalography-study/