Category: Neuropharmacology
Objective: To evaluate the efficacy and safety of 3 doses of DaxibotulinumtoxinA for Injection (DAXI) vs placebo for adults with upper limb spasticity (ULS) after stroke or traumatic brain injury.
Background: Botulinum toxin type A (BoNTA) is first-line treatment for adult ULS; however, symptom re-emergence is reported at ~12 weeks [1]. DAXI, an investigational BoNTA, has shown extended duration of activity in cervical dystonia treatment [2,3].
Method: In this Phase 2, double-blind, placebo-controlled study, adults with ULS were randomized to receive a total dose of DAXI 500U (n=18), 375U (n=19), 250U (n=22), or placebo (n=24) injected into targeted muscles. Follow up was 36 weeks. Co-primary endpoints were Modified Ashworth Scale (MAS) change from baseline in a pre-defined suprahypertonic muscle group (SMG) and Physician Global Impression of Change (PGIC) at Week 6. Duration of effect was defined as time to loss of improvement in SMG (MAS return to baseline) and PGIC ≤0, or subject requesting re-treatment. Study enrollment was reduced due to COVID-19.
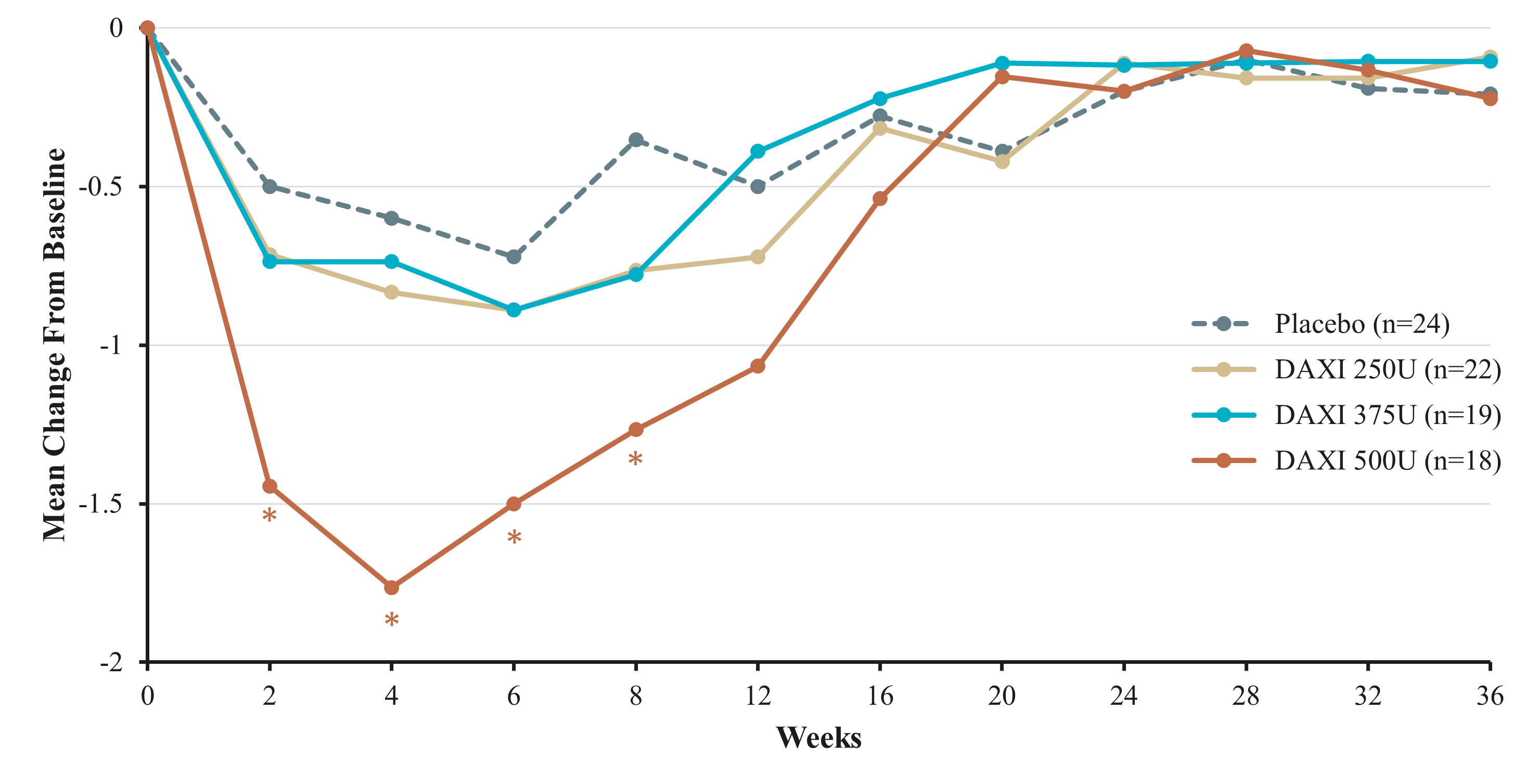
Results: A total of 83 male (59%) and female adults with ULS after stroke (95.2%) or traumatic brain injury (4.8%) were enrolled. DAXI 500U produced a clinically meaningful improvement from baseline in MAS for the SMG at Week 4 (DAXI 500U -1.8 [-46.2%] vs placebo -0.6 [‑15.0%]; p=0.0002) and Week 6 (DAXI 500U -1.5 [-38.5%] vs placebo -0.8 [-20.0%]; p=0.0488) [Figure]. Improvements in MAS for DAXI 250U and 375U at Week 4 (-0.9 [-22.5%] and -0.9 [-22.5%], respectively) and Week 6 (-0.9 [-22.5%] and -1.0 [-25.0%]) did not differ significantly from placebo [Table]. Mean PGIC at Week 4 for DAXI 375U (1.7) and 500U (1.8) was improved vs placebo (0.9; p=0.0150 and p=0.0092, respectively). Median duration of effect was 24.7 weeks in the DAXI 500U group. Analysis of individual muscle groups indicated significant MAS improvement vs placebo at DAXI 100U for the fingers and wrist, whereas change in MAS for the elbow muscle group did not differ significantly from placebo at DAXI 150U. DAXI at doses of 250U, 375U, and 500U was well tolerated, with no observed dose-related increase in adverse events.
Conclusion: Results from this study indicate DAXI at doses of 500U is effective and well tolerated for treating adult ULS. These findings will inform a Phase 3 study design.
References: 1. Jacinto J, et al. Front Neurol. 2020;11:388.
2. Solish N, et al. Drugs. 2021;81(18):2091-2101.
3. Jankovic J, et al. Poster presented at: TOXINS 2021 (virtual).
To cite this abstract in AMA style:
A. Patel, M. Munin, Z. Ayyoub, G. Francisco, T. Gross, R. Rubio, J. Kesslak. DaxibotulinumtoxinA for Injection in Adults With Upper Limb Spasticity After Stroke or Traumatic Brain Injury: A Randomized Placebo-Controlled Study (JUNIPER) [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/daxibotulinumtoxina-for-injection-in-adults-with-upper-limb-spasticity-after-stroke-or-traumatic-brain-injury-a-randomized-placebo-controlled-study-juniper/. Accessed January 31, 2026.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/daxibotulinumtoxina-for-injection-in-adults-with-upper-limb-spasticity-after-stroke-or-traumatic-brain-injury-a-randomized-placebo-controlled-study-juniper/