Category: Rating Scales
Objective: Present the development process and clinimetric testing plan for the International Parkinson and Movement Disorder Society Parkinson’s Disease Psychosis Rating Scale (MDS-PDPRS).
Background: Psychosis affects around 50% of patients with Parkinson’s disease (PD) and contributes to poor patient outcomes and caregiver burden(1). Currently, there are no validated tools for assessing the functional impact of PD psychosis (PDP). Developing a PDP Clinical Outcome Assessment (COA) will help us understand the effects of the PDP symptoms, monitor changes over time and assess the effectiveness of therapeutic interventions in clinical trials.
Method: We used a mixed-methods approach based on best practices for developing an individual level-focused COA for clinical trials(2-4). We involved North American, African, European, and Oceanian native English-speaker research centers. We describe a four-phase multimethod process to conceptualize, develop, quantitatively evaluate, and validate the MDS-PDPRS.
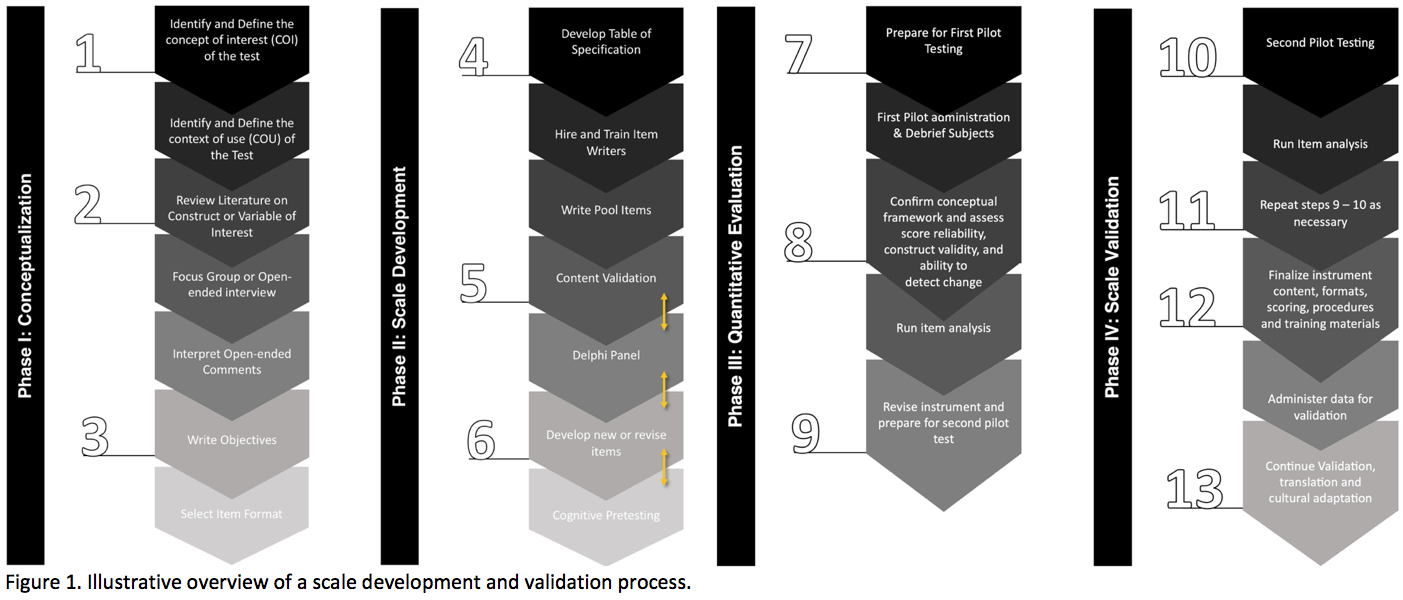
Results: The study is divided into four phases, each with a specific focus and objective: 1) Development of the conceptual framework: This phase aims to establish the conceptual framework that underpins the construction of the MDS-PDPRS by identifying and defining the concept of interest and its context of use. 2) Formalization of the MDS-PDPRS development: This phase aims to formalize the development of the MDS-PDPRS by generating an item pool and implementing cognitive pre-testing to establish face and content validity. 3) Pilot testing and review: This phase aims to conduct a pilot test of the MDS-PDPRS to assess its practicality and identify any areas for improvement. The results of this phase will inform the refinement of the measurement instrument. 4) Clinimetric testing plan: This phase aims to conduct a clinimetric testing plan to evaluate the new measurement instrument’s dimensionality, validity, and reliability, providing a complete picture of the MDS-PDPRS and how it can be used in clinical practices and trials. (Figure 1)
Conclusion: The MDS-PDPRS development plan presented in this report supports the feasibility of creating an instrument to measure the multidimensional components of PDP and addresses the challenges of developing a new COA, with the ultimate goal of creating a valuable tool for precise characterization and measurement of PDP as part of clinical care and trials.
References: 1. Aarsland D, Marsh L, Schrag A. Neuropsychiatric symptoms in Parkinson’s disease. Mov Disord. 2009;24(15):2175-86.
2. US Food and Drug Administration. Guidance for Industry Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims [Internet]. 2009 [cited 2021 Aug 10]. p. 1–39.
3. Boateng GO, Neilands TB, Frongillo EA, Melgar-Quiñonez HR, Young SL. Best Practices for Developing and Validating Scales for Health, Social, and Behavioral Research: A Primer. Front Public Health. 2018;6:149.
4. Sampaio C, Goetz CG, Schrag A. Rating Scales in Parkinson’s Disease: clinical practice and research. New York: Oxford University Press; 2012. 342 p.
To cite this abstract in AMA style:
M. Tosin, T. Mestre, G. Stebbins, G. Mangone, S. Videmsky, S. Ali, D. Aarsland, J. Goldman, T. Khoo, S. Lewis, P. Martinez-Martin, O. Ojo, J. Pagonabarraga, A. Schrag, D. Weintraub, C. Goetz. Development and Clinimetric Testing Plan for the MDS Parkinson’s Disease Psychosis Rating Scale (MDS-PDPRS) [abstract]. Mov Disord. 2023; 38 (suppl 1). https://www.mdsabstracts.org/abstract/development-and-clinimetric-testing-plan-for-the-mds-parkinsons-disease-psychosis-rating-scale-mds-pdprs/. Accessed February 24, 2026.« Back to 2023 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/development-and-clinimetric-testing-plan-for-the-mds-parkinsons-disease-psychosis-rating-scale-mds-pdprs/