Session Information
Date: Tuesday, September 24, 2019
Session Title: Rating Scales
Session Time: 1:45pm-3:15pm
Location: Les Muses Terrace, Level 3
Objective: To develop and validate the PD-MAS.
Background: Parkinson’s disease (PD) is a neurodegenerative disease manifested by motor and non-motor symptoms, controlled with drug, neurosurgical and rehabilitation therapies. Regarding drug therapy, adherence is an important clinical construct for two major reasons. First, problems with adherence is common, with evidence that only 10% of people with PD adhere adequately to the prescribed treatment. Second, inadequate use of the medications can be an important issue that compromise the quality of life of the patient. Although medication adherence is a readily accepted clinical construct, it represents a complex phenomenon that is difficult to define and measure objectively, since it is dependent on the interrelationship of intentional and unintentional factors. Given the importance of the construct and the inherent difficulties in its definition and quantification, it is important to have a clinical instrument that measures medication adherence in PD.
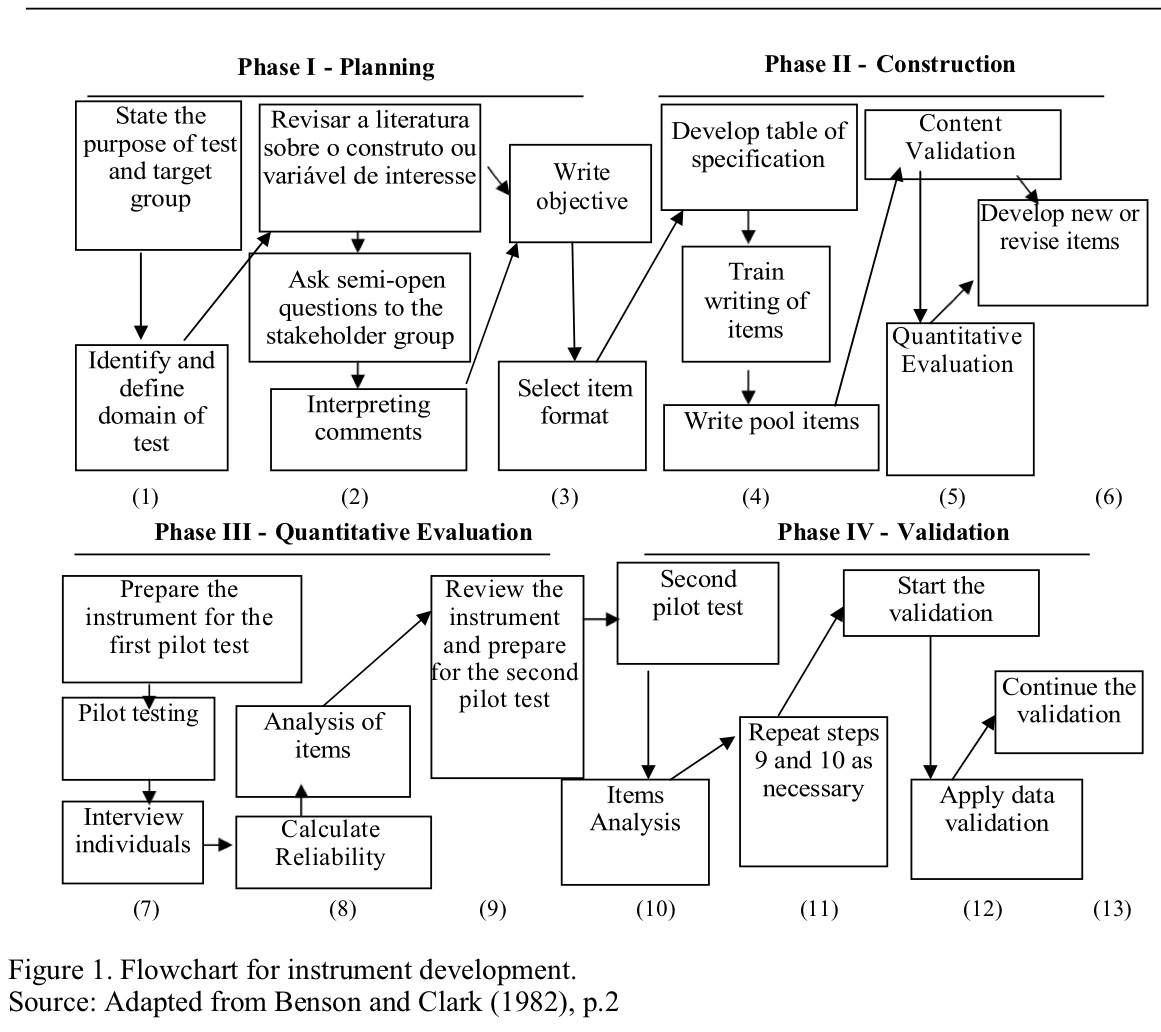
Method: This is a methodological and psychometric study for the development of a valid, accurate and reliable scale that will be developed based on the recommendations of Benson & Clark (1982), in 4 phases and up to thirteen steps (Figure 1). To date, this research concludes step 1 and, in part, step 2.
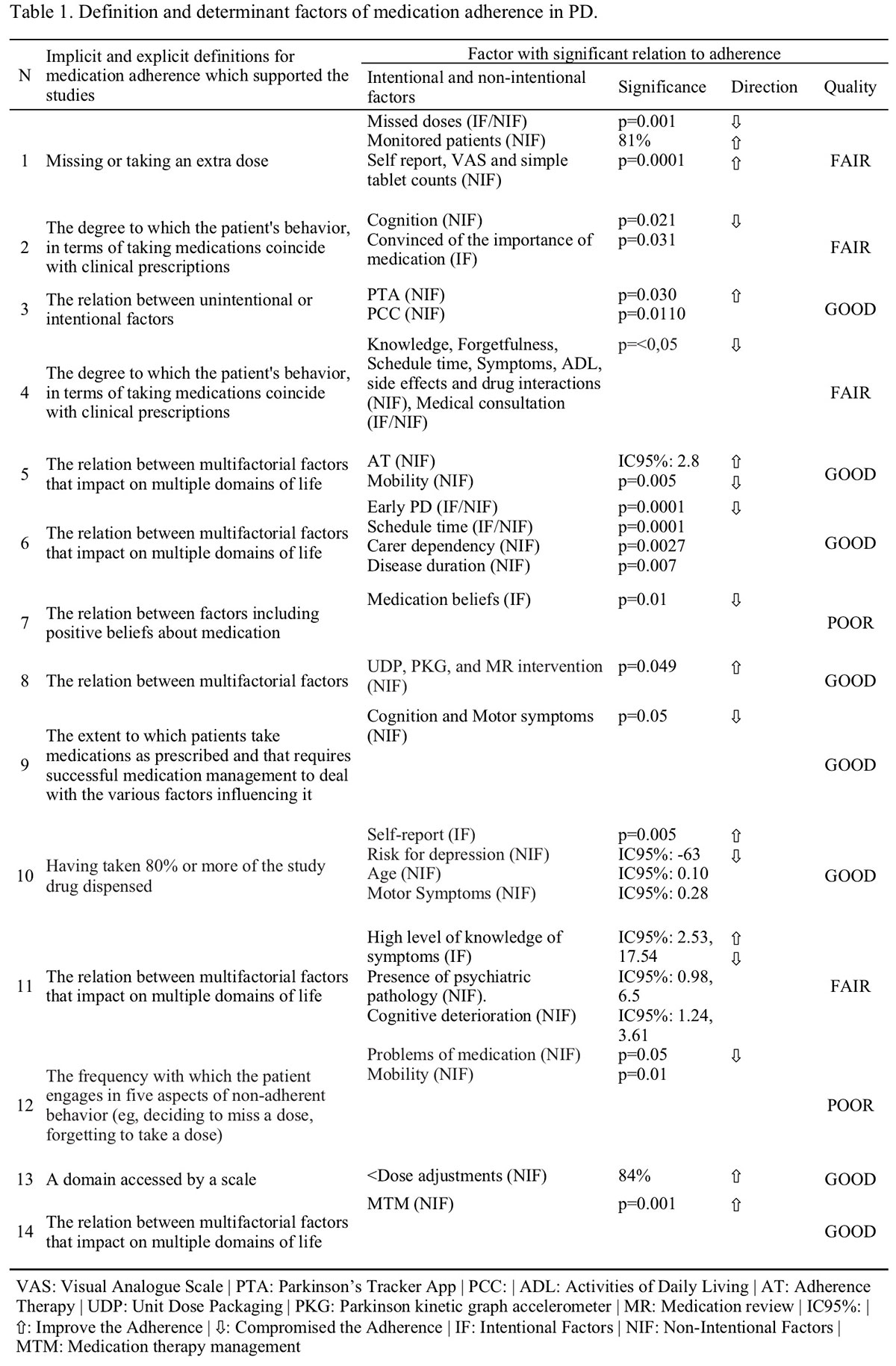
Results: As a result of step 1 we defined:a) population of interest and the objectives: to measure the medication adherence in people with PD.b) global domain: adherence to antiparkinsonian medication.c) content areas: intentional and unintended factors of medication adherence. The first refers to the intentional behaviors of the patient, family member and/or caregiver not to follow or follow partially the prescribed treatment. The second, or unintentional, refers to any barrier that does not depend on the individual.d) question to be answered: what are the factors of medication adherence in PD?e) purposes of the measurement items: to measure the influence of the factors of medication adherence during the last month.f) restrictions on use: cognitive impairment.g) procedures for the use of the instrument: still under construction. Step 2 is underway, and a systematic review has been completed for the identification of observable and relevant phenomena in the global domain of interest (Table 1).
Conclusion: The research is in progress, and until now it has been concluded that the factors of medication adherence are diverse in PD.
References: 1. Leopold NA, Polansky M, Hurka MR. Drug adherence in Parkinson’s disease. Mov Disord. 2004;19(5):513–7. 2. Fabbrini G, Abbruzzese G, Barone P, Antonini A, Tinazzi M, Castegnaro G, et al. Systematic review on factors associated with medication non-adherence in Parkinson’s disease. Int J Clin Pract [Internet]. Elsevier Ltd; 2012;18(2):963–71. 3. Benson J, Clarck F. A guide for instrument development and validation. The American journal of occupational therapy: official publication of the American Occupational Therapy Association. 1982;36(12): 789–800.
To cite this abstract in AMA style:
M. Tosin, B. Oliveira. Development and Validation of the Parkinson’s Disease Medication Adherence Scale (PD- MAS) [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/development-and-validation-of-the-parkinsons-disease-medication-adherence-scale-pd-mas/. Accessed February 8, 2026.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/development-and-validation-of-the-parkinsons-disease-medication-adherence-scale-pd-mas/