Category: Parkinson’s Disease: Clinical Trials
Objective: To assess the efficacy and safety of foslevodopa/foscarbidopa (LDp/CDp) in people with Parkinson’s (PwP) with and without care partner support.
Background: The progression of Parkinson’s disease (PD) often leads to increased symptom burden and treatment complexity. While PwP may benefit from care partner support, it is unclear if care partners improve outcomes in those receiving continuous subcutaneous infusion (CSCI) of LDp/CDp.
Method: This post hoc analysis was conducted in patients with advanced PD who received LDp/CDp in a 12-week, phase 3, randomized controlled trial (NCT04380142). Patients were ≥30 years old, had levodopa-responsive idiopathic PD, and were inadequately controlled (≥2.5 “Off” hours/day) by current oral therapy. Patients and/or care partners must have shown correct use of the CSCI delivery system and were required to provide informed consent before enrollment. Change from baseline to final visit in “On” time, “Off” time, 39‑item Parkinson’s Disease Questionnaire (PDQ-39), and Movement Disorder Society‑Unified Parkinson’s Disease Rating Scale Part II (MDS-UPDRS II) were assessed in subgroups of patients with and without care partner support.
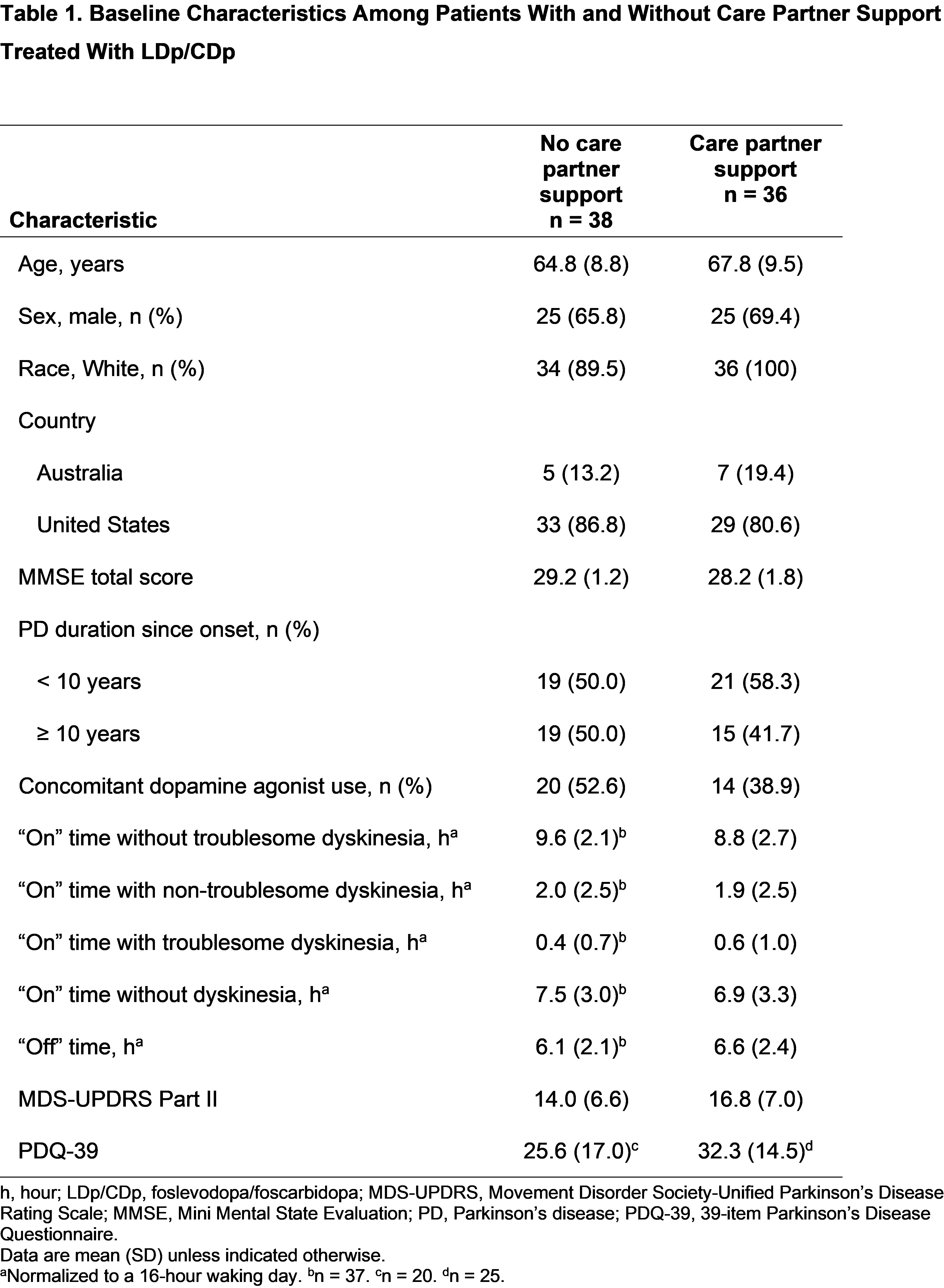
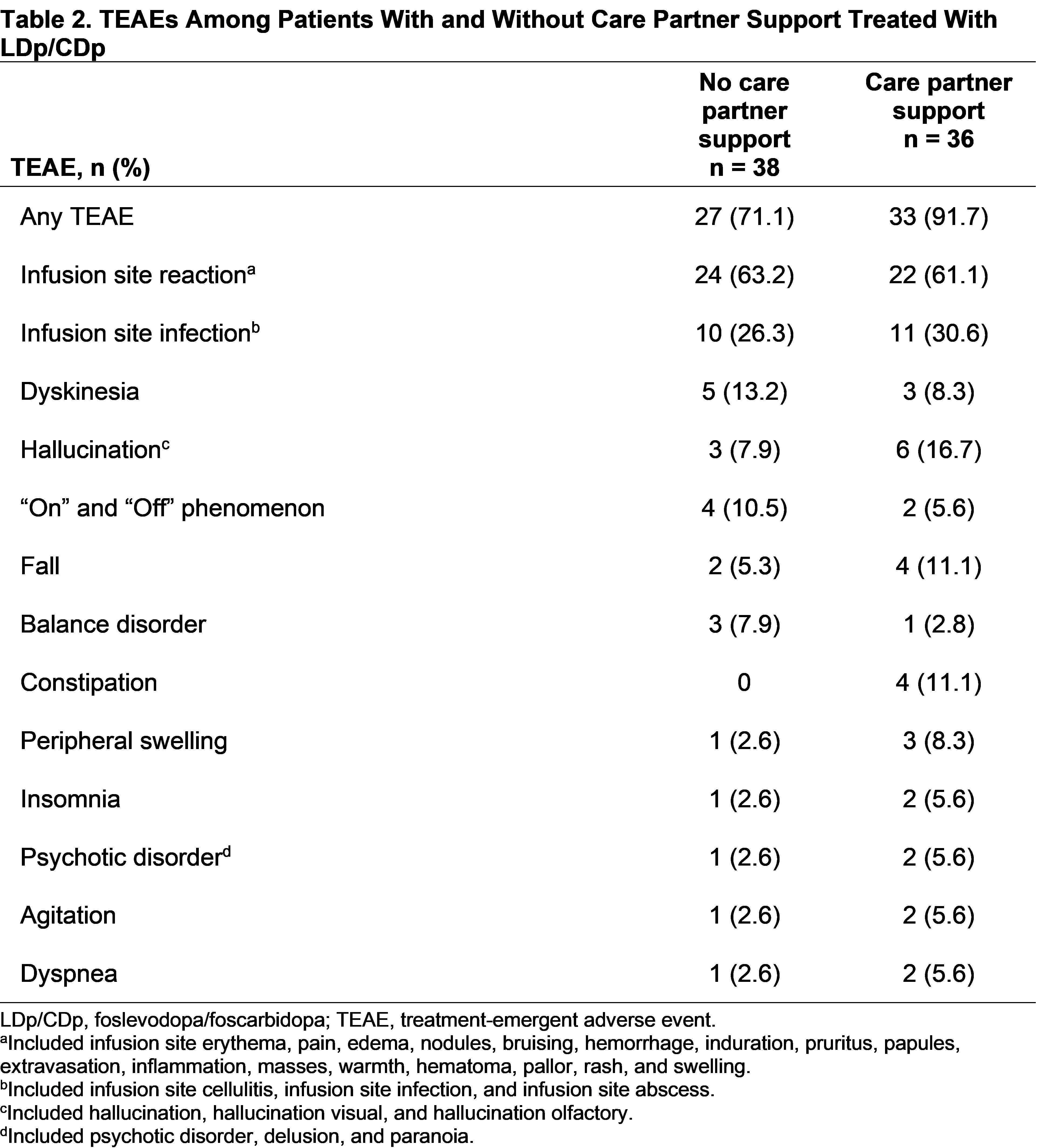
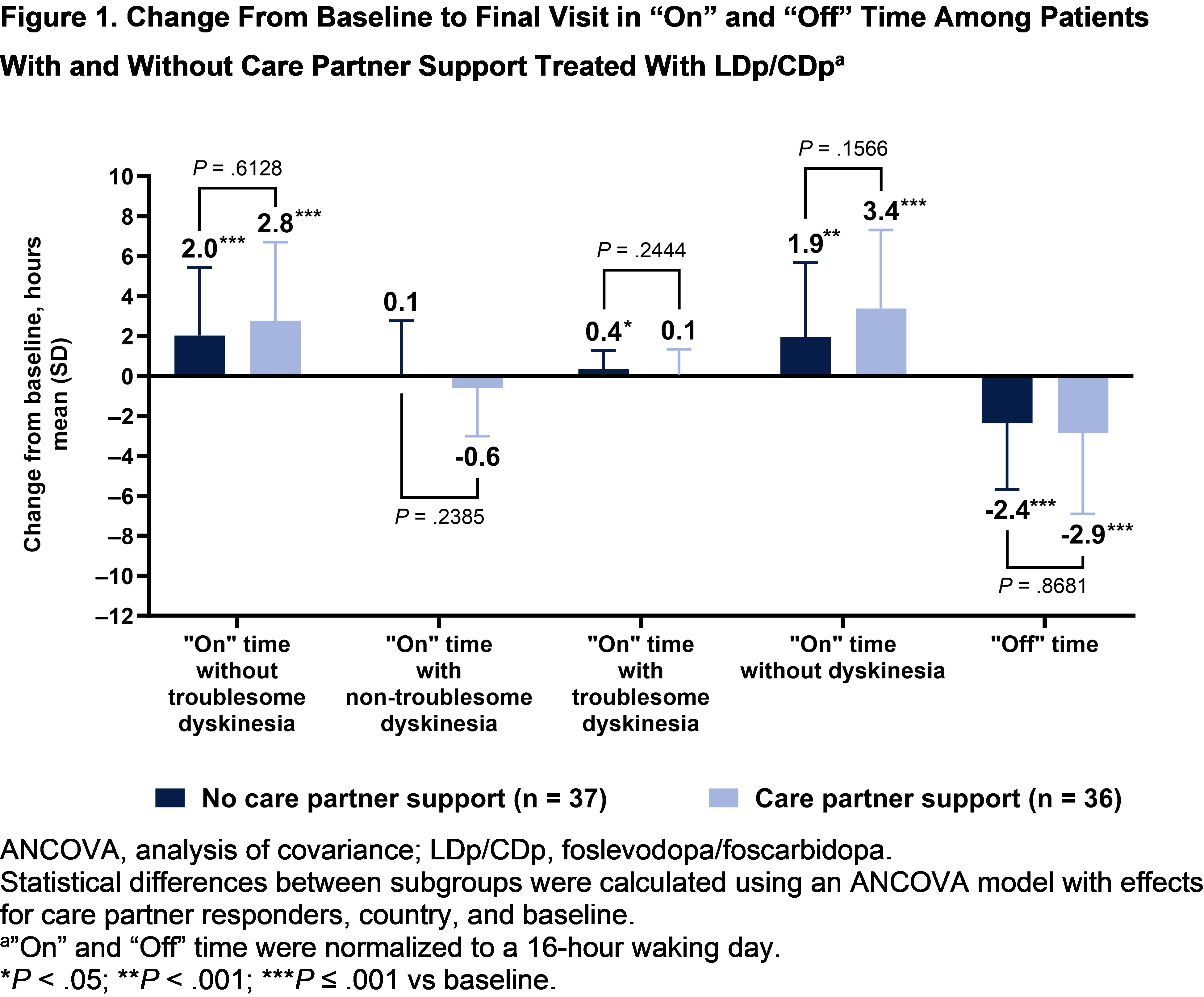
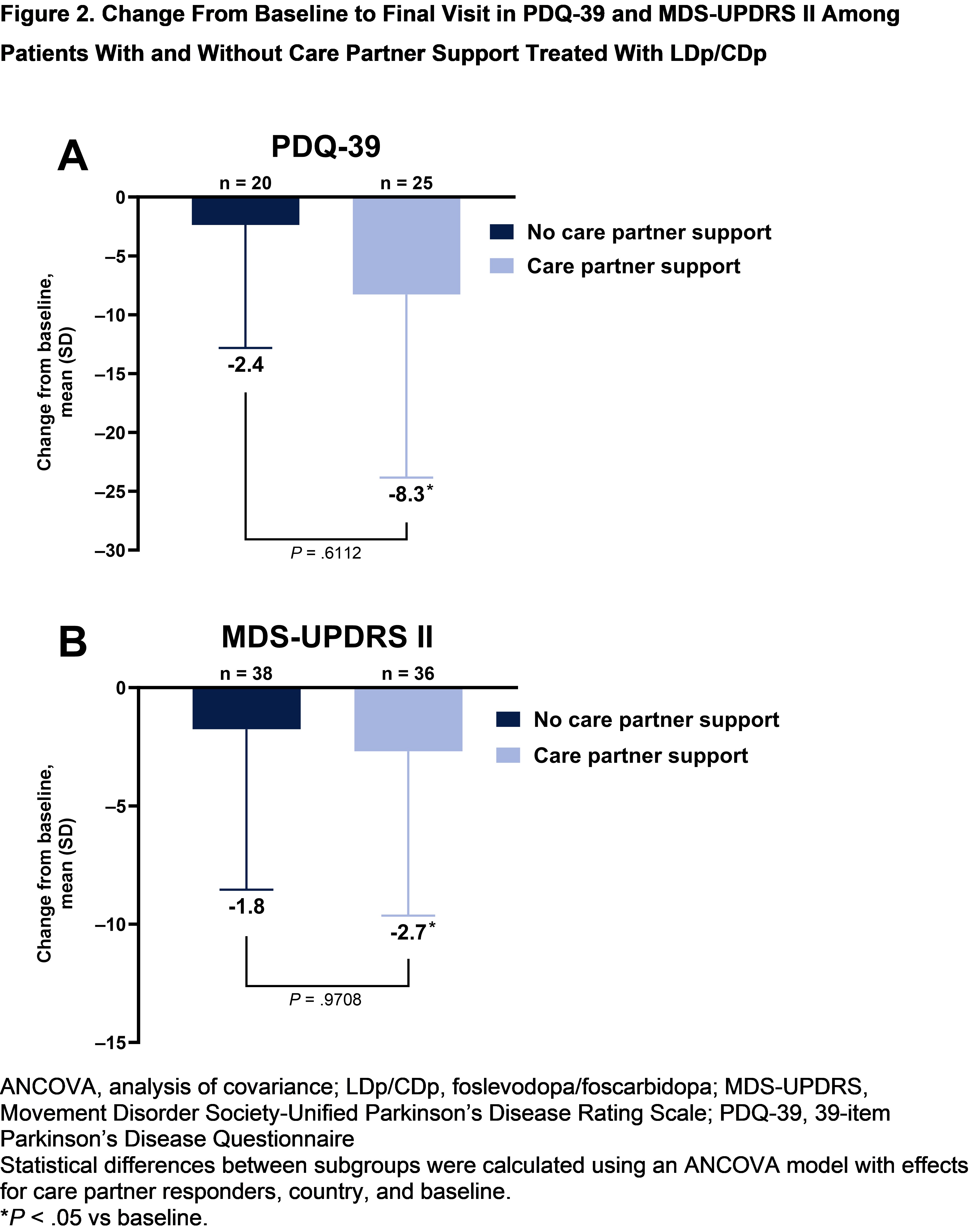
Results: Among 74 patients randomized to LDp/CDp, 36 (48.6%) had an enrolled care partner. Baseline characteristics were similar across subgroups, except concomitant dopamine agonist use was numerically greater in patients without care partner support [table1]. Patients in both subgroups experienced improvements from baseline in “On” time without troublesome dyskinesia, “On” time without dyskinesia, and “Off” time (P<.001) [figure1]. Patients with care partner support experienced improvements from baseline in PDQ‑39 and MDS-UPDRS II scores (P<.05); patients without care partner support did not [figure2]. However, no significant differences were observed between subgroups for all assessed efficacy outcomes. Overall rates of TEAEs were numerically higher in patients with vs without care partner support (91.7% vs 71.1%) [table2]. Rates of infusion site infections and reactions were similar between subgroups.
Conclusion: There were no differences in the efficacy and safety of LDp/CDp among PwP with and without care partner support, suggesting that those with advanced PD can achieve comparable outcomes with LDp/CDp irrespective of care partner support. These results may not apply to those with high cognitive impairment.
Table 1
Table 2
Figure 1
Figure 2
To cite this abstract in AMA style:
M. Soileau, J. Bronstein, L. Harmer, M. Shah, R. Gupta, L. Verhagen Metman. Efficacy and Safety of Foslevodopa/Foscarbidopa in People With Parkinson’s Disease With and Without Care Partner Support [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-foslevodopa-foscarbidopa-in-people-with-parkinsons-disease-with-and-without-care-partner-support/. Accessed July 10, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/efficacy-and-safety-of-foslevodopa-foscarbidopa-in-people-with-parkinsons-disease-with-and-without-care-partner-support/