Session Information
Date: Saturday, October 6, 2018
Session Title: Clinical Trials and Therapy in Movement Disorders
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: We describe the quality of life outcome from an open-label trial of daxibotulinumtoxinA for injection (Daxi) in cervical dystonia (CD) as measured by the Cervical Dystonia Impact Profile-58 (CDIP-58), a disease-specific patient reported outcome measure.
Background: The efficacy and safety of Daxi, a neuromodulator comprised of 150 kDa BoNTA and a proprietary stabilizing excipient RTP004, in patients with CD have been described (1). This report focuses on the patient derived assessments of quality of life using the CDIP-58 following Daxi.
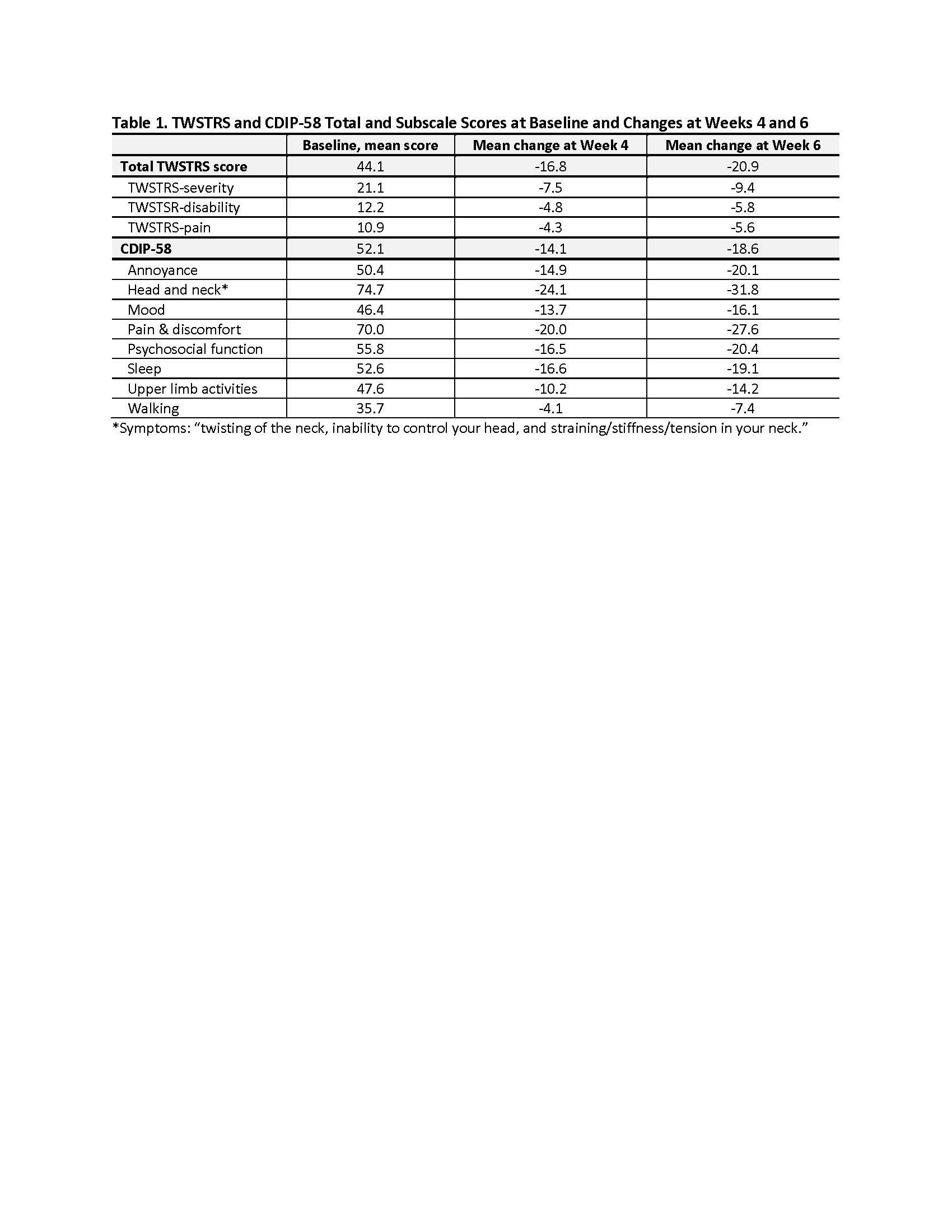
Methods: Adults with CD (total Toronto Western Spasmodic Torticollis Scale [TWSTRS] score ≥20 and TWSTRS-severity score ≥15), BoNT-naïve or previously treated, were enrolled and given one treatment of Daxi. The primary efficacy endpoint was change in total TWSTRS score at Week 4. Secondary efficacy endpoints included change in CDIP-58 total and subscale scores and duration of treatment effect. The CDIP-58 questionnaire was administered at baseline and Weeks 4, 6, 12, 16, 20, and 24.
Results: 37 adults (mean age: 56 years, 76% female, mean CD duration: 7.6 years, 46% BoNT-experienced) were administered one IM injection of daxiBoNTA, mean dose (range): 244 (100 – 450) units. Maximal reductions in TWSTRS and CDIP-58 total and subscale scores were observed at Week 6 (Table 1). The greatest improvement in symptom burden (relative to baseline) was observed for the head and neck symptoms at Week 6 (-43%), followed by annoyance (-40%), pain and discomfort (-39%). There was sustained benefit (mean reduction [absolute, relative]) based on total TWSTRS [-12.8, -29%] and CDIP-58 [-12.6, -24%]) maintained through Week 24. The median duration of treatment effect, defined as time from initial injection to retreatment request or loss of 80% of the treatment benefit (reduction in total TWSTRS achieved at Week 4), was 25.3 weeks. Treatment-related dysphagia occurred in 5 (14%) subjects (all mild in severity and completely resolved; mean duration: 35 days). Daxi was well-tolerated and generally safe; there were no serious AEs.
Conclusions: Treatment of CD with daxibotulinumtoxinA for injection resulted in improvement of symptoms and quality of life that was most notable at Week 6 with benefit sustained through Week 24. These results warrant further confirmation in larger phase 3 studies.
References: Jankovic J, Truong D, Patel A, et al. Injectable DaxibotulinumtoxinA in Cervical Dystonia: A Phase 2 Dose-Escalation Multicenter Study. Movement Disorder Clinical Practice (accepted, Feb 2018).
To cite this abstract in AMA style:
C. Comella, A. Brashear, J. Jankovic, D. Truong, A. Patel, M. Evatt, N. Kapa, Y. Liu, T. Nguyen-Cleary. Improvement in Patient Perceived Quality of Life with DaxibotulinumtoxinA for Injection in Adults with Cervical Dystonia [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/improvement-in-patient-perceived-quality-of-life-with-daxibotulinumtoxina-for-injection-in-adults-with-cervical-dystonia/. Accessed July 10, 2025.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/improvement-in-patient-perceived-quality-of-life-with-daxibotulinumtoxina-for-injection-in-adults-with-cervical-dystonia/