Category: Parkinsonism, Atypical: PSP, CBD
Objective: PSP-P is the second most common PSP phenotype. To facilitate its inclusion in future clinical trials, we used longitudinal data from the TAUROS study [1] to calculate a power estimate specific to this PSP phenotype.
Background: The progression rate of PSP-P is slower than the classic PSP-Richardson syndrome (PSP-RS) [2]. This hinders inclusion of this phenotype in the clinical trials. Power estimation for this phenotype will hopefully facilitate this inclusion. We used data from TAUROS trial for this estimation. This dataset benefits from longitudinal evaluation of PSP-P patients in whom phenotypes were assigned prospectively, using the Williams criteria [3], since there were no standard operationalized PSP-P criteria at the time.
Method: We included subjects with baseline and 52-week follow-up data available. Effect size was calculated based on mean and standard deviation for each outcome measure at baseline and at week 52. Sample size calculation was then performed based on the obtained effect size for parametric and non-parametric analysis at significance level of 0.05 and power of 80%. Placebo effect has previously been assessed and ruled out for this dataset by Stamelou et al [4].
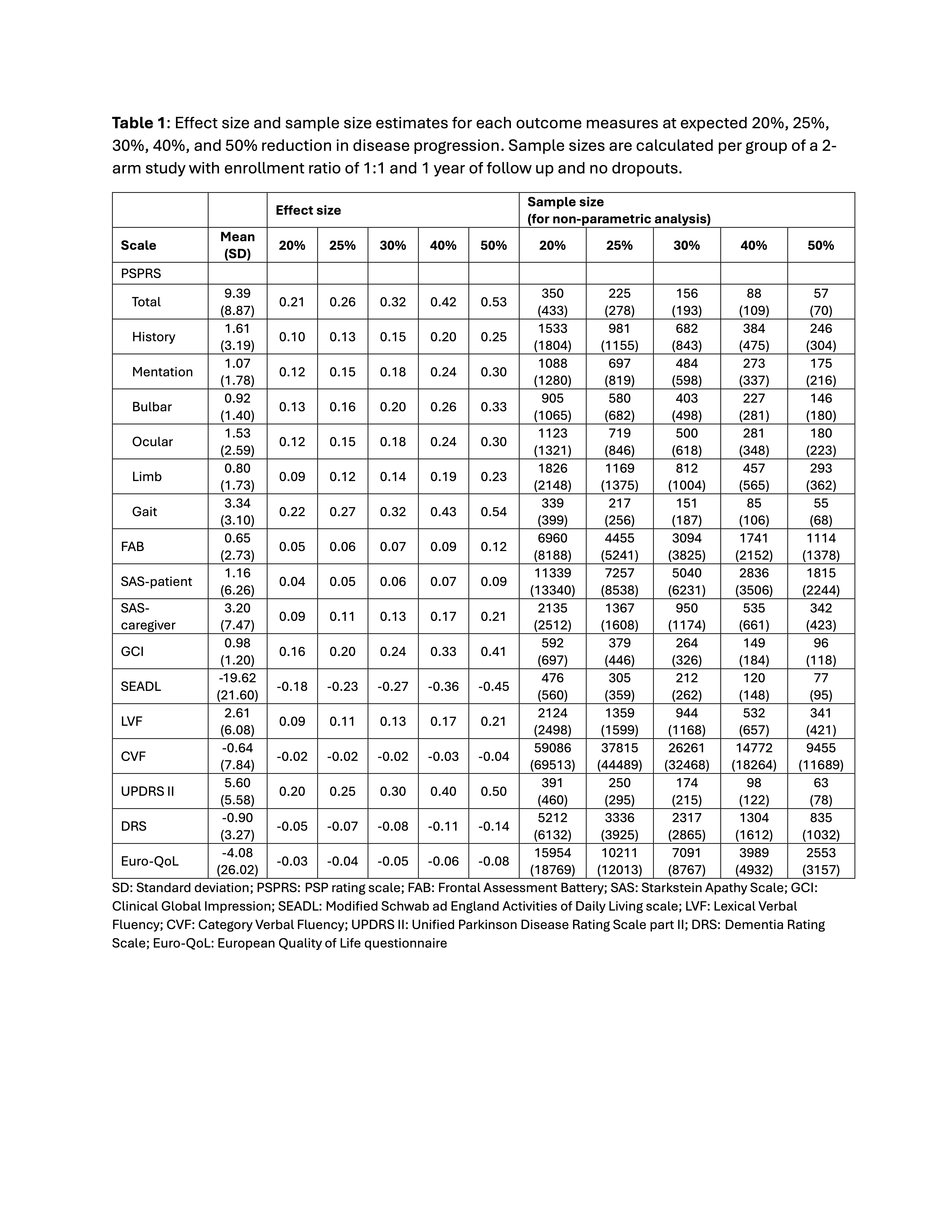
Results: A maximum of 55 PSP-P patients were included in the analysis, 30 male and 25 female, with a mean age of 70.24 (SD= 6.58) and mean disease duration of 3.44 (SD=4.28) years. Calculated effect size and sample size are shown in Table 1. Samples of 57, 88, and 156 subjects per group would be needed to find a 50%, 40%, or 30% reduction in progression of the total PSP rating scale (PSPRS) score, respectively. The annual difference in the total PSPRS score was 9.39 (SD=8.86) which is lower than that of PSP-RS phenotype (11.24±9.95). The dropout rate was 19% (13/68), lower compared to PSP-RS phenotype (26%). Adjustment for this dropout rate gives a sample size of 70 (57/0.81), 109, and 193 per group for the PSPRS total score with an expected 50%, 40%, or 30% reduction, respectively.
Conclusion: Atypical PSP phenotypes have been known for more than 20 years; however, therapeutic clinical trials have avoided including them so far due to multiple factors including lack of knowledge about the behavior of these phenotypes as well as diagnostic challenges. Sample size estimation for these other phenotypes is an important step that allows inclusion of these patients to the clinical trials.
Table 1
References: [1] Tolosa E, Litvan I, Höglinger GU, Burn D, Lees A, Andrés MV, Gómez-Carrillo B, León T, Del Ser T; TAUROS Investigators. A phase 2 trial of the GSK-3 inhibitor tideglusib in progressive supranuclear palsy. Mov Disord. 2014 Apr;29(4):470-8. doi: 10.1002/mds.25824.
[2] Shoeibi A, Litvan I, Tolosa E, Ser TD, Lee E; TAUROS Investigators. Progression of two Progressive Supranuclear Palsy phenotypes with comparable initial disability. Parkinsonism Relat Disord. 2019 Sep;66:87-93. doi: 10.1016/j.parkreldis.2019.07.010.
[3] Williams DR, de Silva R, Paviour DC, Pittman A, Watt HC, Kilford L, Holton JL, Revesz T, Lees AJ. Characteristics of two distinct clinical phenotypes in pathologically proven progressive supranuclear palsy: Richardson’s syndrome and PSP-parkinsonism. Brain. 2005 Jun;128(Pt 6):1247-58. doi: 10.1093/brain/awh488.
[4] Stamelou M, Schöpe J, Wagenpfeil S, Del Ser T, Bang J, Lobach IY, Luong P, Respondek G, Oertel WH, Boxer A, Höglinger GU; AL-108-231 Investigators, Tauros Investigators, and MDS-Endorsed PSP Study Group. Power calculations and placebo effect for future clinical trials in progressive supranuclear palsy. Mov Disord. 2016 May;31(5):742-7. doi: 10.1002/mds.26580.
To cite this abstract in AMA style:
N. Olfati, S. Akhlaghi, A. Shoeibi, I. Litvan. Inclusion of PSP-Parkinsonism (PSP-P) phenotype in clinical trials – power calculation [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/inclusion-of-psp-parkinsonism-psp-p-phenotype-in-clinical-trials-power-calculation/. Accessed February 3, 2026.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/inclusion-of-psp-parkinsonism-psp-p-phenotype-in-clinical-trials-power-calculation/