Objective: To examine the real-world long-term effectiveness of carbidopa/levodopa enteral suspension (CLES) on off time and other patient reported outcomes (PROs) among advanced Parkinson’s disease (APD) patients in USA
Background: CLES, continuous infusion of carbidopa/levodopa delivered via percutaneous endoscopic gastrojejunostomy (PEG-J) and a portable pump, is used to treat motor fluctuations among APD patients. Recent studies have demonstrated the efficacy of CLES in significantly reducing off time up to 3-5 years of treatment initiation [1]. However, US-specific data on long-term effectiveness of CLES is still emerging.
Method: PROviDE is a prospective, observational, real-world study [2]. All APD patients participating in the patient support program were eligible for this study. PROs were collected every 6-months over a period of 36 months. The last subject last visit is scheduled in July 2021. This abstract focuses on off-time (primary endpoint) and treatment satisfaction (secondary endpoint) using interim 36-month follow-up data; the final data including other exploratory PROs such as sleep, dyskinesia, quality of life, and fatigue will be presented during the conference. Descriptive statistics and paired t-tests were used to summarize the data.
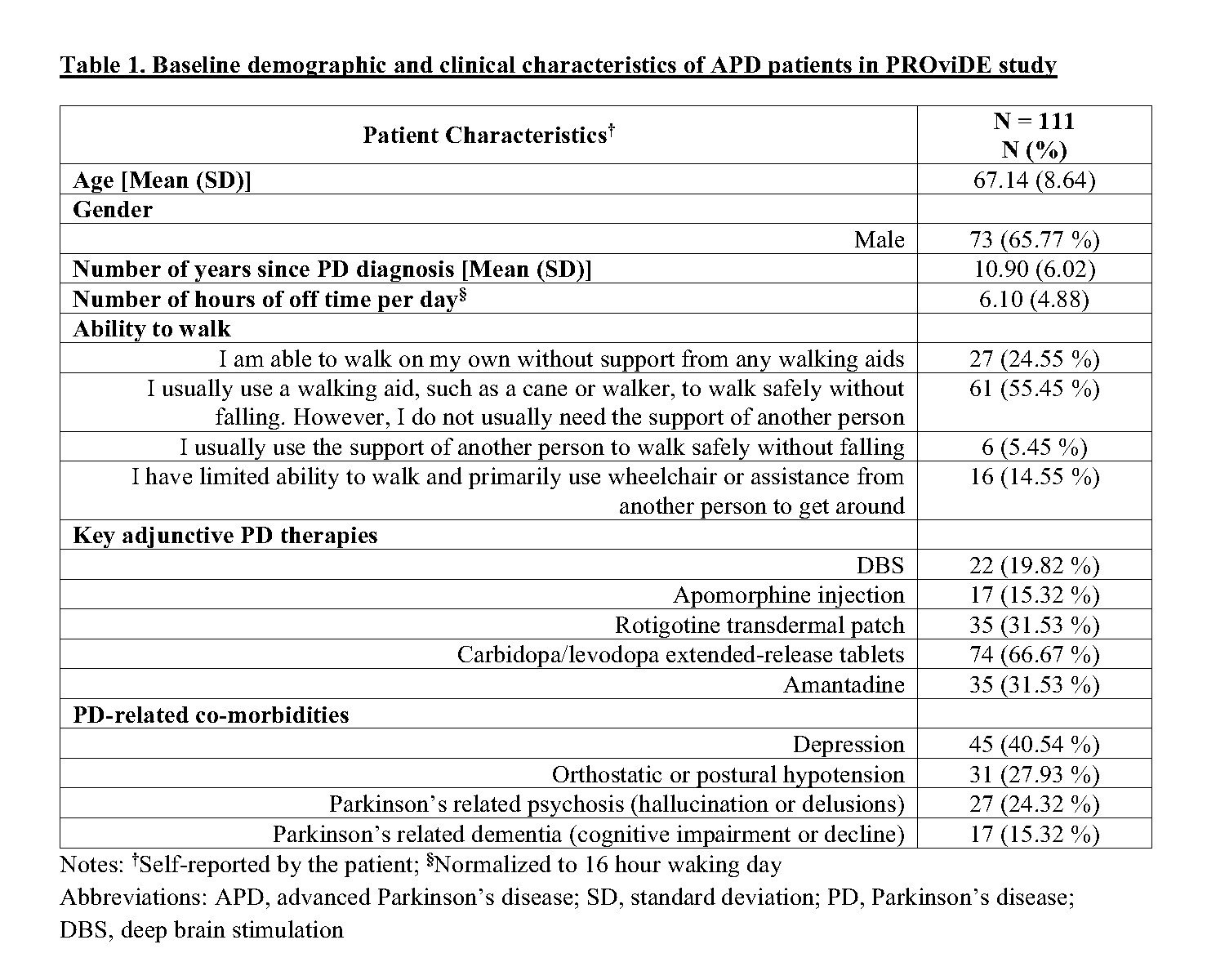
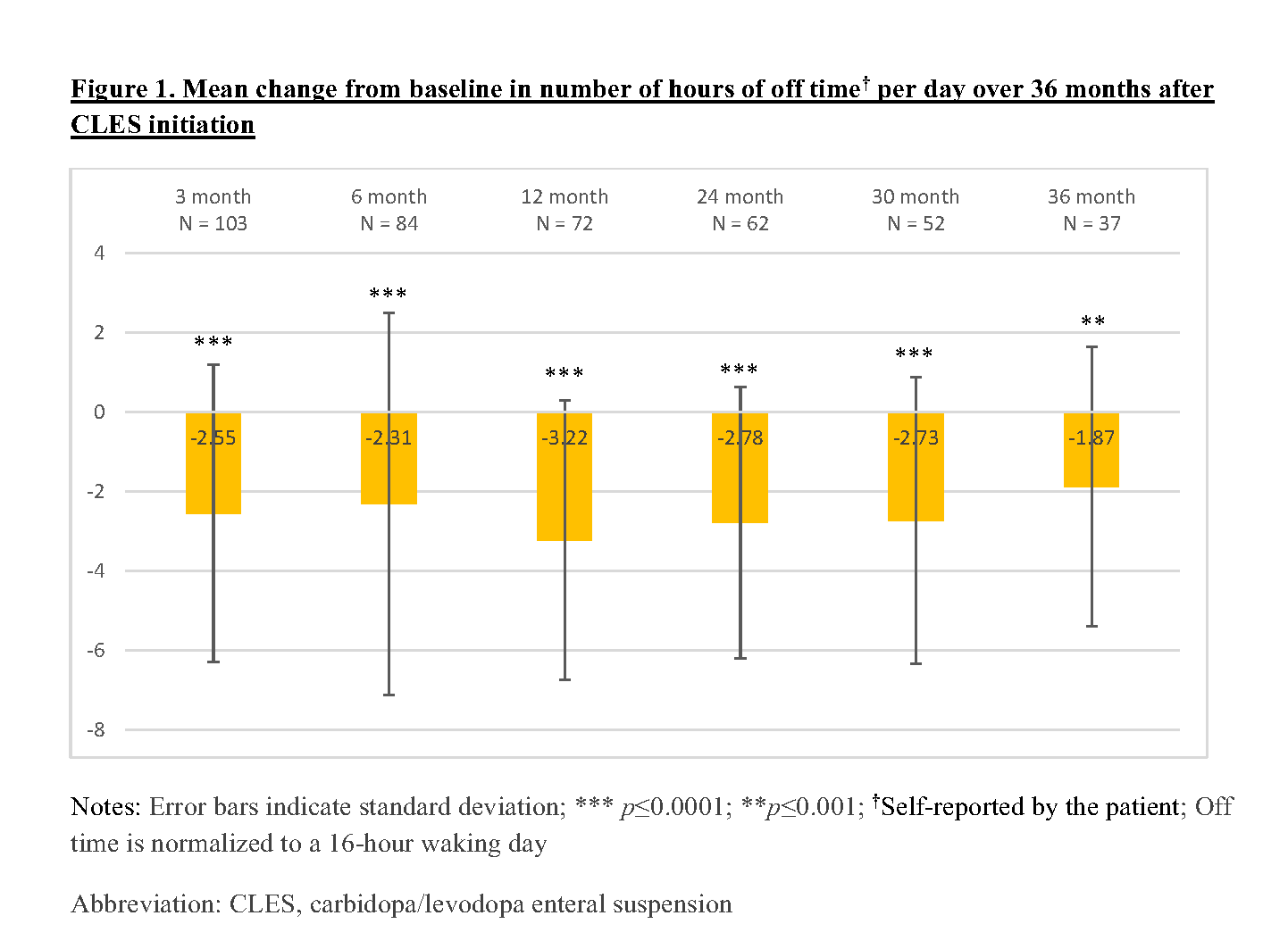
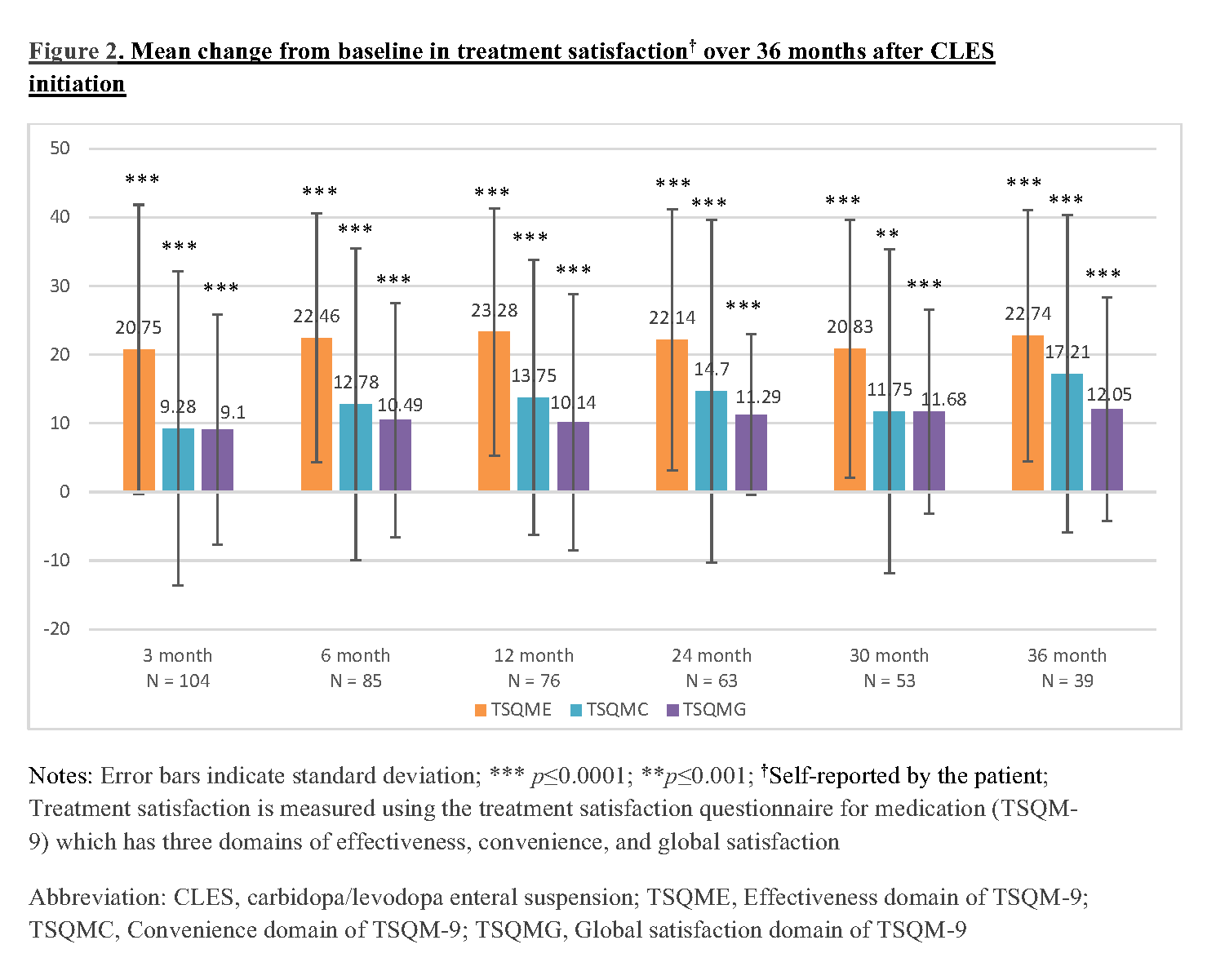
Results: A sample of 111 APD patients who initiated CLES were recruited in this study. The baseline characteristics of these patients are described in Table 1. Of these 111 patients, 68.5% and 56.8% completed the 12-month and 24-month assessments, respectively, and remained on CLES therapy. About 13.5% of the recruited patients were deceased and 21.62% discontinued CLES by 24 months. In this interim analysis, patients demonstrated sustained reduction (p<0.05) in the number of hours of off time per day [Figure 1] and sustained improvement (p<0.05) in treatment satisfaction with effectiveness, convenience, and global satisfaction [Figure 2] over a period of 36 months after CLES initiation.
Conclusion: This interim analysis demonstrates the effectiveness of CLES in achieving about 2 times the minimal clinically important change in off time (i.e.,1 hour) [3] within 3 months of treatment initiation and sustaining the effect over 36 months along with improved treatment satisfaction. Final data for all patients and adjusted estimates are warranted to further examine the effectiveness of CLES.
References: 1. Antonini, A., Odin, P., Pahwa, R., Aldred, J., Alobaidi, A., Jalundhwala, Y., … & Chaudhuri, K. (2019). Long-term impact of Levodopa Carbidopa Intestinal Gel (LCIG) on reducing “Off” time: Results from a systematic review of studies with≥ 12 months of follow-up: 60. Movement Disorders, 34: S1-S930. https://doi.org/10.1002/mds.27795 2. Pahwa, R., Dorsey, R., Kandukuri, P., Bao, Y., Zamudio, J., Pan, I., & Jalundhwala, Y. (2018). Evaluating long-term effectiveness of carbidopa/levodopa enteral suspension in advanced Parkinson’s Disease patients: PROviDE study design and baseline characteristics. Movement Disorders, 33, pp. S93-S94. https://doi.org/10.1002/mds.116 3. Hauser, R. A., Auinger, P., & Parkinson Study Group. (2011). Determination of minimal clinically important change in early and advanced Parkinson’s disease. Movement Disorders, 26(5), 813-818.
To cite this abstract in AMA style:
R. Pahwa, S. Isaacson, R. Dorsey, D. Cella, B. Bluett, S. Thakkar, P. Kandukuri, N. Gupta, Y. Jalundhwala, Y. Bao, I. Pan, O. Ladhani, J. Aldred. Long-term real-world effectiveness of carbidopa/levodopa enteral suspension after 36 months of treatment initiation: Final results from PROviDE study [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/long-term-real-world-effectiveness-of-carbidopa-levodopa-enteral-suspension-after-36-months-of-treatment-initiation-final-results-from-provide-study/. Accessed July 15, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/long-term-real-world-effectiveness-of-carbidopa-levodopa-enteral-suspension-after-36-months-of-treatment-initiation-final-results-from-provide-study/