Objective: To describe a series of patients under treatment with subcutaneous foslevodopa/foscarbidopa, either as initiation therapy or after switching from intestinal levodopa/carbidopa.
Background: Treatment with subcutaneous foslevodopa/foscarbidopa offers a new possibility in the management of Parkinson’s disease uncontrolled by conventional therapy. Studies describe an improvement in fluctuations, motor, and non-motor symptoms, but real-life studies are scarce due to its recent commercialization.
Method: Thirteen patients were recruited according to standard clinical practice for initiation of foslevodopa/foscarbidopa (DUODOPA PS). The STAT-ON device was used for assessing fluctuations and progression (pre- and post-therapy), along with clinical interviews, motor assessment (part III-UPDRS), quality of life (PDQ-39), and non-motor symptoms (PDNMS-Q).
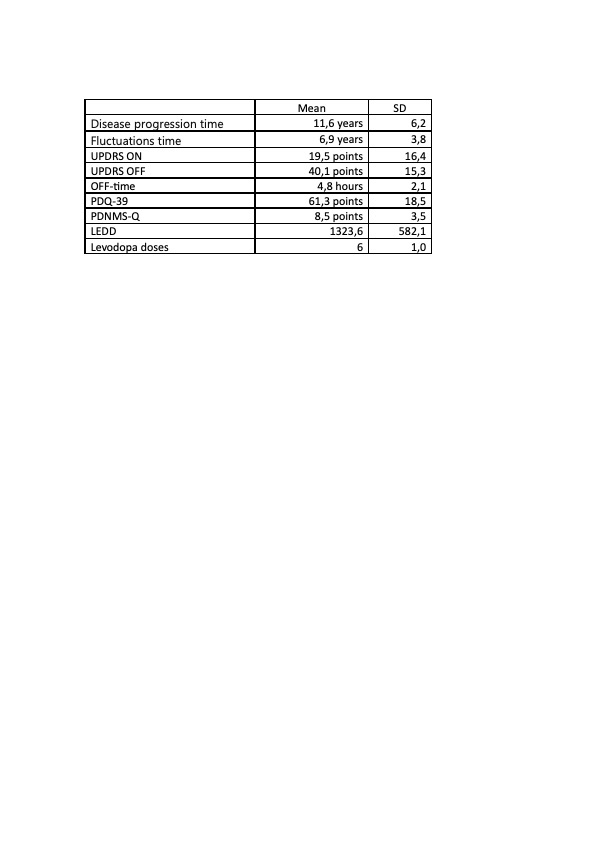
Results: Thirteen patients were recruited, two of whom had previous treatment with Duodopa® intestinal gel. Sixty-nine percent were male. Mean age was 67.8 years (SD 11.02). Clinical characteristics of Parkinson’s disease prior to treatment are outlined in Table 1. Eighty-nine percent of patients had dyskinesias, with 44% being disruptive. Thirty percent of patients had mild cognitive impairment, none had dementia. Seventy-seven percent of patients had a caregiver (mostly their spouse). Fifty-four percent had previous concomitant treatment with dopaminergic agonists, 46% with MAO-B inhibitors, and 38% with COMT inhibitors. Initiation of foslevodopa/foscarbidopa was done on an movement disorders outpatient clinic, with a mean of 13 days until optimization, without severe complications in any patient. After optimization, improvement in motor symptoms, quality of life and non-motor symptoms was observed. The majority of patients achieved monotherapy. STAT-ON records showed improvement in OFF time and fluctuations.
Conclusion: Subcutaneous foslevodopa/foscarbidopa treatment offers a safe, effective, and straightforward alternative for optimization in patients with Advanced Parkinson’s Disease.
Tabla 1
References: Soileau MJ, Aldred J, Budur K, Fisseha N, Fung VS, Jeong A, Kimber TE, Klos K, Litvan I, O’Neill D, Robieson WZ, Spindler MA, Standaert DG, Talapala S, Vaou EO, Zheng H, Facheris MF, Hauser RA. Safety and efficacy of continuous subcutaneous foslevodopa-foscarbidopa in patients with advanced Parkinson’s disease: a randomised, double-blind, active-controlled, phase 3 trial. Lancet Neurol. 2022 Dec;21(12):1099-1109. doi: 10.1016/S1474-4422(22)00400-8. Erratum in: Lancet Neurol. 2023 Mar;22(3):e5. PMID: 36402160.
Rosebraugh M, Voight EA, Moussa EM, Jameel F, Lou X, Zhang GGZ, Mayer PT, Stolarik D, Carr RA, Enright BP, Liu W, Facheris MF, Kym PR. Foslevodopa/Foscarbidopa: A New Subcutaneous Treatment for Parkinson’s Disease. Ann Neurol. 2021 Jul;90(1):52-61. doi: 10.1002/ana.26073. Epub 2021 May 4. PMID: 33772855; PMCID: PMC8251848.
Rosebraugh M, Liu W, Neenan M, Facheris MF. Foslevodopa/Foscarbidopa Is Well Tolerated and Maintains Stable Levodopa and Carbidopa Exposure Following Subcutaneous Infusion. J Parkinsons Dis. 2021;11(4):1695-1702. doi: 10.3233/JPD-212813. PMID: 34366380; PMCID: PMC8609688.
To cite this abstract in AMA style:
M. Morales-Casado, G. Tabar-Comellas, E. Gisbert-Tijeras, DD. García-Meléndez, J. Domínguez-Bértalo, D. Rivero-Rodríguez, N. López-Ariztegui. Multicenter study of efficacy and safety of subcutaneous foslevodopa/foscarbidopa treatment initiation or switch from LD/CD intestinal gel [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/multicenter-study-of-efficacy-and-safety-of-subcutaneous-foslevodopa-foscarbidopa-treatment-initiation-or-switch-from-ld-cd-intestinal-gel/. Accessed July 15, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/multicenter-study-of-efficacy-and-safety-of-subcutaneous-foslevodopa-foscarbidopa-treatment-initiation-or-switch-from-ld-cd-intestinal-gel/