Session Information
Date: Wednesday, June 22, 2016
Session Title: Rating scales
Session Time: 12:00pm-1:30pm
Location: Exhibit Hall located in Hall B, Level 2
Objective: To design and validate a quantitative method for the assessment of PKAN patients.
Background: Despite advances in the research of novel therapies for PKAN, validated clinical rating scales are lacking.
Methods: Design of a Disease Rating Scale for PKAN (PKAN-DRS) including cognitive, behavioral, Parkinsonian, dystonic, other neurological signs and disability. Items were scored from 0 (normal) to 3 or 4 (maximum severity)(total scores 0-155). Two examiners assessed patients at different centres following a video-recording protocol. Then, three independent examiners rated patients using the recorded videotapes (only spasticity and rigidity were rated in live).
Results: The PKAN-DRS was applied to 45 PKAN patients (age onset 12±9 years; age assessment 30±17 years) from 17 centers in Europe. Median (IQR) values for the total scale were 71(42-85). Age at disease onset correlated with age at loss of independent gait (r=,771) and with ratings of cognitive impairment (r=-0.447), dystonia (r=-,484), disability (r=-,582) and total scale (r=-,532) (Spearman). Age at assessment showed positive correlation with parkinsonism (r=,402) and negative with dystonia (r=,394). Regarding parkinsonism, patients showed mild to severe (2 to 4 score) postural instability (70%), global hypokinesia (53%), and rigidity (31%), whereas rest tremor was only detected in four patients (0.1%). Mild to severe (2 to 4 score) dystonia was present in >50% of the 10 body parts, preventing chewing (41%), standing (30%) and useful grasp (20%). The ICCs for inter-observer agreement was considered “almost perfect” (ICC=0.975), with an internal reliability considered as “good” (Cronbach’s alpha =0.83). Regarding inter-item evaluation, reliability was considered “good” for dystonia, parkinsonism and disability sub-scores (0.90, 0.86 and 0.91, respectively), and “poor” for other neurological signs (gait, speech and oculomotor abnormalities)(0.68). Eight homozygous patients for the common c.1583C>T mutation in PANK2 showed a more benign phenotype with lower PKAN-DRS scores 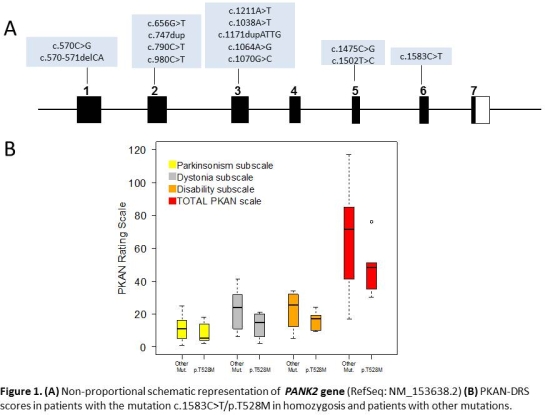 .
.
Conclusions: The proposed PKAN-DRS seems a reliable method for the assessment of PKAN. Generalized dystonia with prominent jaw opening/cleching is a prominent sign, together with hypokinesia, postural instability and rigidity. The earlier the onset of disease, the higher the ratings are. With increasing age, Parkinsonian features are more severe whereas dystonia is less prominent.
To cite this abstract in AMA style:
A. Darling, C. Garrido, S. Aguilera, M. Tomas-Vila, I. Gaston, M. Madruga, L. González-Gutiérrez, J. Ramos-Lizana, M. Pujol, K. Tustin, J.P. Lin, L. Martorell, C. Tello, V. Lupo, C. Espinos, L. Stefanis, L. Sanz, F. Gutiérrez, P.J. Garcia, L. Vela, T. Temudo, R. Pons, M.J. Martí, B. Pérez-Dueñas. Pantotenate kinase associated neurodegeneration (PKAN): Proposal for a clinical rating scale [abstract]. Mov Disord. 2016; 31 (suppl 2). https://www.mdsabstracts.org/abstract/pantotenate-kinase-associated-neurodegeneration-pkan-proposal-for-a-clinical-rating-scale/. Accessed March 1, 2026.« Back to 2016 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/pantotenate-kinase-associated-neurodegeneration-pkan-proposal-for-a-clinical-rating-scale/
