Category: Parkinson’s Disease: Clinical Trials
Objective: To assess the long-term safety and tolerability of pimavanserin in frail older adults and elderly patients with neuropsychiatric symptoms related to a neurodegenerative disease (NDD).
Background: Pimavanserin, a 5-HT2A and, to a lesser extent, 5-HT2C receptor inverse agonist/antagonist, is the only medication FDA-approved to treat hallucinations and delusions associated with Parkinson’s disease (PD) with or without dementia.
Expanding safety knowledge in frail patients with NDDs, including PD, is helpful for informing its use in PD psychosis, given these patients are highly sensitive to the adverse effects (AEs) of antipsychotics.
Method: This 52-week, multicenter, Phase 3b, open-label (OL) extension study evaluated the long-term safety of 34 mg once daily pimavanserin in adult and elderly patients with neuropsychiatric symptoms related to NDDs. Patients from an antecedent double-blind (DB) study of pimavanserin enrolled; both placebo (DB PBO-OL PIM) and pimavanserin (DB PIM-OL PIM) groups from that study received pimavanserin in this OL extension. Treatment emergent AEs (TEAEs) were the primary endpoint; exploratory endpoints included other safety measures (Extrapyramidal Symptom Rating Scale-Abbreviated, ESRS-A; Mini-Mental State Examination, MMSE) and efficacy analyses focused on neuropsychiatric symptoms, health-related quality of life, and sleep quality. Descriptive summaries are provided.
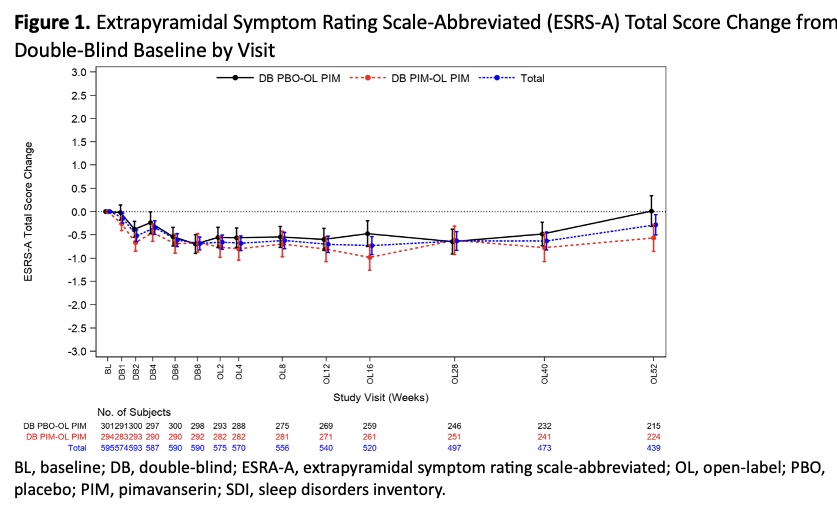
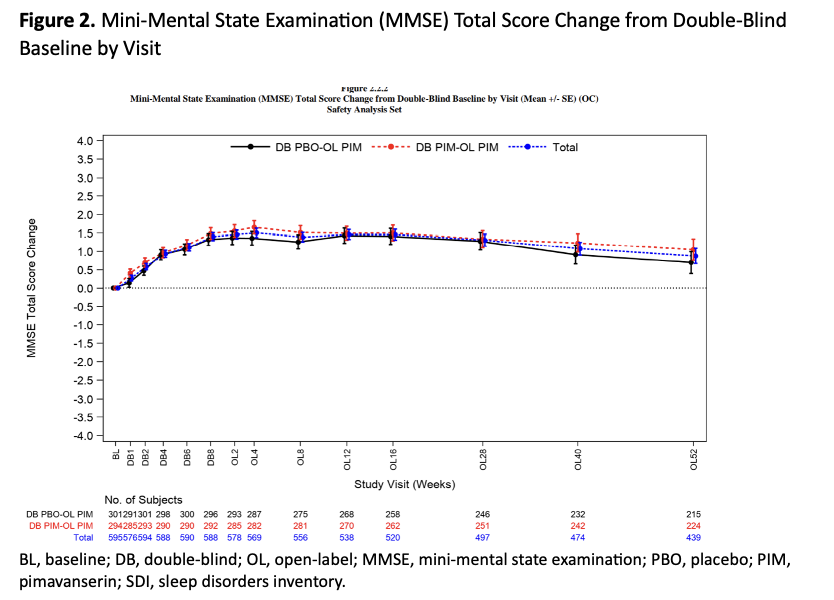
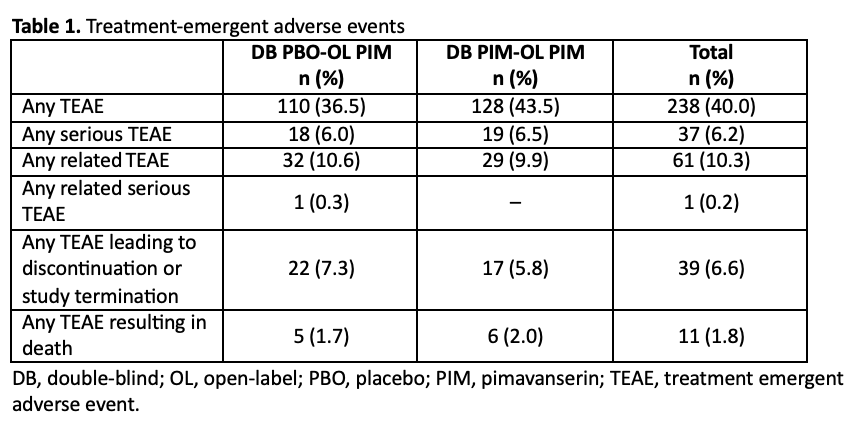
Results: Overall, 595 patients were treated; 452 (76.0%) completed the study. The mean patient age was 72.2 years, 58.5% of patients were female, and 94.3% were white. 95.3% of patients had diagnosed dementia; the most common causes of dementia were Alzheimer’s disease (68.7%), vascular dementia (18.5%), and PD (4.7%). The mean duration of exposure to pimavanserin was 312.4 days. Overall, 40% of patients experienced a TEAE (Table 1). Serious TEAEs occurred in 6.2% of patients, TEAEs leading to study discontinuation occurred in 6.6%, and 11 patients (1.8%) experienced a TEAE leading to death. No clinical impairment versus placebo were seen in motor function (ESRS-A; Figure 1) or cognition (MMSE; Figure 2).
Conclusion: Our results show that pimavanserin was generally well tolerated in older adult and frail elderly patients with neuropsychiatric symptoms related to a NDD. No new safety signals were observed.
Figure 1
Figure 2
Table 1
To cite this abstract in AMA style:
W. Cubała, A. Berrio, K. Chi-Burris, G. Alva, L. Chrones, S. Pathak. Pimavanserin Safety in Adult and Elderly Patients With Neuropsychiatric Symptoms Related to Neurodegenerative Disease: an Open-Label Extension Study [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/pimavanserin-safety-in-adult-and-elderly-patients-with-neuropsychiatric-symptoms-related-to-neurodegenerative-disease-an-open-label-extension-study/. Accessed July 14, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/pimavanserin-safety-in-adult-and-elderly-patients-with-neuropsychiatric-symptoms-related-to-neurodegenerative-disease-an-open-label-extension-study/