Objective: To describe (1) the real-world effectiveness of the multidisciplinary inpatient rehabilitation (MIR) ‘Parkinson’s Disease Multimodal Complex Treatment’ (PD-MCT) in people with PD (PwP) compared to outpatient care-as-usual, (2) predictors of a positive or absent effect, and (3) aspects of health care utilization (HCU).
Background: MIR has been associated with improved health-related quality of life (QoL), activities of daily living, motor and non-motor symptoms of PwP. In Germany, more than 200 hospitals perform multidisciplinary PD care within the framework of PD-MCT. Although widely implemented and increasingly applied, PD-MCT has not been evaluated by randomized, controlled trials (RCT) until now.
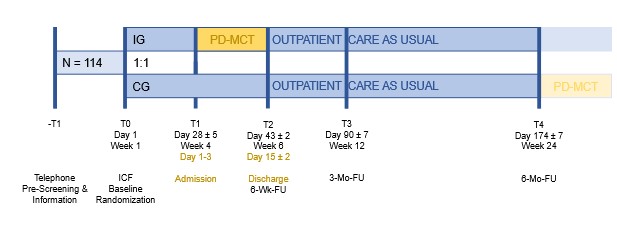
Method: This is a pragmatic, single-center, 1:1-randomized, open-label, parallel-group, wait-list controlled study on a two-week multidisciplinary inpatient treatment vs. outpatient care-as-usual. 114 participants with PD and disease stage HY <5 will be enrolled. The intervention group receives PD-MCT four weeks after T0. It includes pharmacological adjustment and non-pharmacological therapies for at least 7.5 h/w (e.g., physiotherapy, occupational therapy, speech and language therapy). The control group receives a PD-MCT appointment after T4 and outpatient care-as-usual for the 6-month duration of the study including appointments at a PD outpatient clinic. Assessments are performed at baseline (T0) and at four follow-up visits (T1 to T4), 4, 6, 12, and 24 weeks after T0 [figure1]. The primary endpoint is the mean change in QoL (PDQ-39) at T2 vs. T0. Secondary endpoints include motor, non-motor, and digital mobility assessments, caregiver burden, costs, HCU, and biological measures. Descriptive and inferential statistics including linear models and machine learning will be applied.
Results: The study was approved by the local ethics committee (23-7828), is registered with the German Clinical Trials Register (DRKS00032619) and funded by the State of North Rhine-Westphalia, Germany (IF-019-22). Recruitment of participants has started in September 2023 and will continue until mid-2025.
Conclusion: This is the first RCT on a kind of multidisciplinary inpatient PD rehabilitation widely applied in Germany. Knowledge of the short- and long-term effects of PD-MCT and a detailed description of the target group may guide future decisions of clinicians and public health managers.
Study Design Chart
To cite this abstract in AMA style:
R. Scherbaum, E. Hinrichs, A. Horstmann, J. Schmidt-Fleischer, J. Stein, J. Geritz, J. Welzel, E. Kwon, J. Oppermann, V. Tschentscher, A. Mattukat, S. Muhlack, S. Faissner, C. Hansen, K. Winklhofer, R. Gold, W. Maetzler, N. Timmesfeld, L. Tönges. ProACT Study Protocol: a Randomized, Wait-list Controlled, Pragmatic Study on Parkinson’s Disease Multimodal Complex Treatment [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/proact-study-protocol-a-randomized-wait-list-controlled-pragmatic-study-on-parkinsons-disease-multimodal-complex-treatment/. Accessed July 15, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/proact-study-protocol-a-randomized-wait-list-controlled-pragmatic-study-on-parkinsons-disease-multimodal-complex-treatment/