Category: Pediatric Movement Disorders
Objective: To present analyses of pooled data on the safety of incobotulinumtoxinA (INCO) in pediatrics
Background: BotulinumtoxinA (BoNT-A) is used to treat several conditions affecting children/adolescents, including spasticity and sialorrhea (excessive drooling). Because these conditions often require repeated, long-term therapy, safety is paramount when treating young people with BoNT-A. INCO, the most purified BoNT-A formulation, is unique in that it is free of all non-toxic accessory proteins [1].
Method: This was a pooled analysis of safety data from four phase 3 studies of children/adolescents (2–17 years) investigating INCO either for the treatment of lower limb and/or upper limb spasticity associated with cerebral palsy [Treatment with IncobotulinumtoxinA in Movement (TIM), Treatment with IncobotulinumtoxinA in Movement Open-label (TIMO), incobotulinumtoXinA in aRm treatment in cerebral pAlsy (XARA)] or sialorrhea associated with neurological disorders [Sialorrhea Pediatric Xeomin Investigation (SIPEXI)]. Figure 1 [figure1] shows INCO doses and number/frequency of injection cycles (ICs). Methodological details can be found in [2–5]. Safety endpoints included treatment-emergent adverse events (TEAEs), treatment-related TEAEs, treatment-related serious adverse events (TESAEs), treatment-related adverse events of special interest (TEAESIs) possibly indicative of toxin spread, and TEAEs leading to discontinuation. All INCO dose groups were combined.
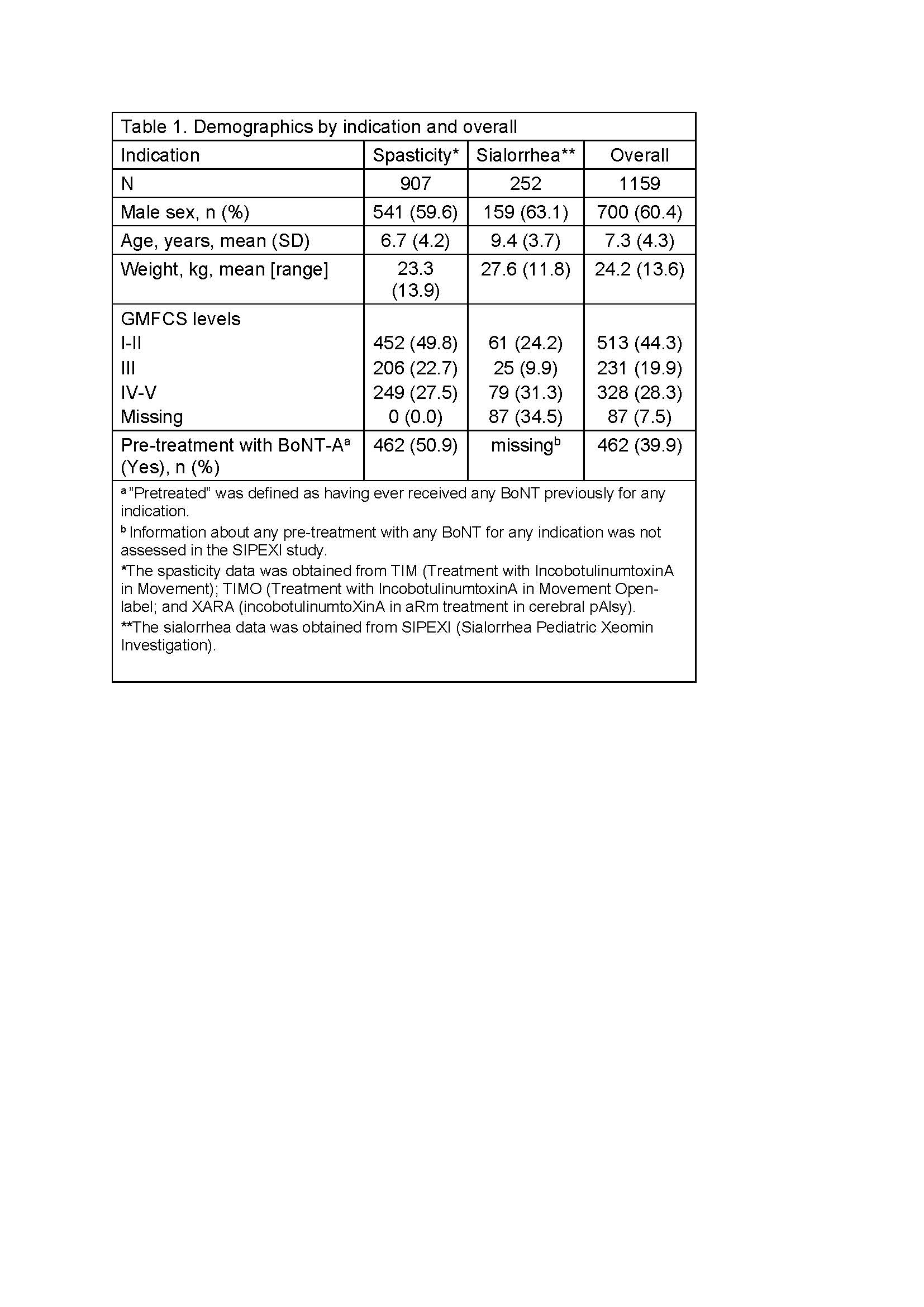
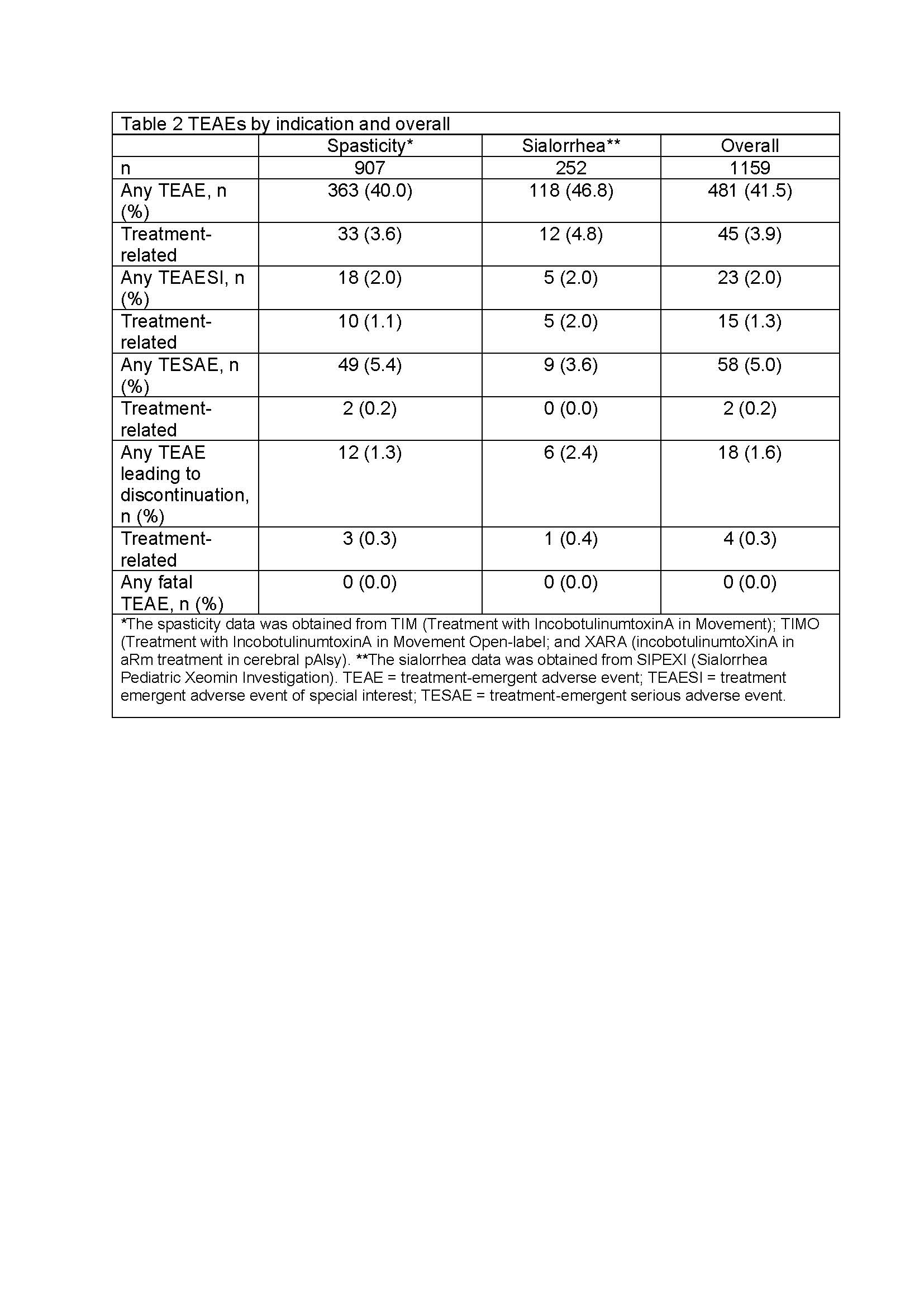
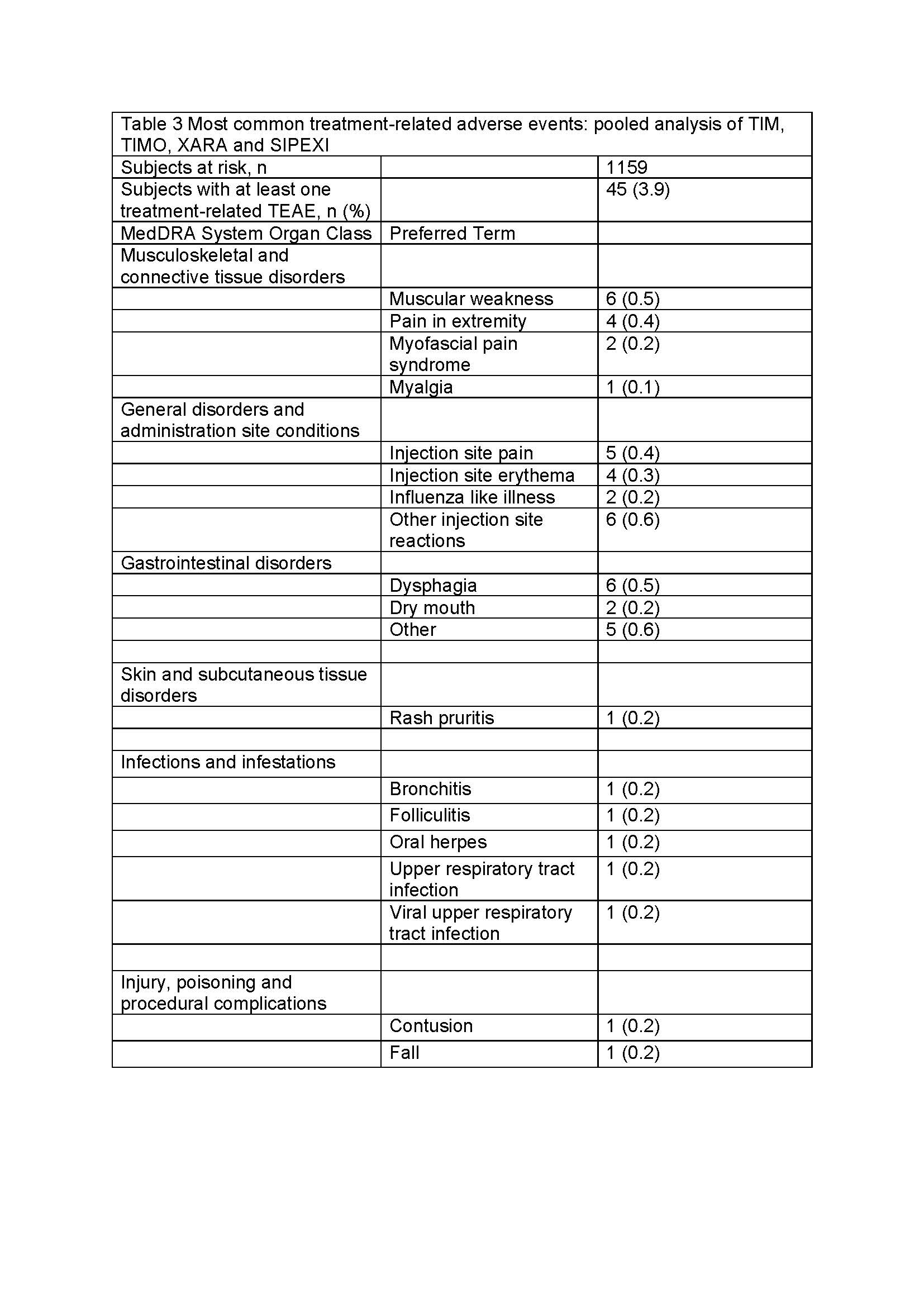
Results: 1159 patients (mean age 7.3 years, 60.4% males, GMFCS levels I-V) were treated with INCO [Table 1]. Patients were treated for 2–6 ICs over ≤96 weeks. Overall, 481 (41.5%) experienced a TEAE. Of these, 45 (3.9%) were considered treatment related by investigators [Table 2], with the most common being injection site reactions (1.3%), muscular weakness (0.5%), and dysphagia (0.5%) [Table 3], and all generally of mild-to-moderate intensity. Two (0.2%) patients with spasticity experienced a treatment-related TESAE and overall 4 (0.3%) discontinued due to treatment-related TEAEs. Safety event frequencies were similar for both indications.
Conclusion: INCO, given repeatedly for up to 96 weeks, was associated with very few treatment-related TEAEs in more than 1100 children/adolescents representing a broad range of age and GMFCS levels. Choosing a BoNT-A with a good safety profile is an important consideration when treating children/adolescents with chronic conditions requiring continuing therapy.
References: 1. Kerscher M, Wanitphakdeedecha R, Trindade de Almeida A, Maas C, Frevert J. IncobotulinumtoxinA: A Highly Purified and Precisely Manufactured Botulinum Neurotoxin Type A. J Drugs Dermatol. 2019;18(1):52-57.
2. Heinen F, Kaňovský P, Schroeder AS, Chambers HG, Dabrowski E, Geister TL, et al. IncobotulinumtoxinA for the treatment of lower-limb spasticity in children and adolescents with cerebral palsy: A Phase 3 study. J Pediatr Rehabil Med. 2021;14(2):183-97. doi. 10.3233/PRM-210040.
3. Kaňovský P, Heinen F, Schroeder AS, Chambers HG, Dabrowski E, Geister TL, et al. Safety and efficacy of repeat long-term incobotulinumtoxinA treatment for lower limb or combined upper/lower limb spasticity in children with cerebral palsy. J Pediatr Rehabil Med (in press), 2021. doi: 10.3233/PRM-210041.
4. Dabrowski E, Chambers HG, Gaebler-Spira D, Banach M, Kaňovský P, Dersch H, et al. IncobotulinumtoxinA efficacy/safety in upper-limb spasticity in pediatric cerebral palsy: Randomized controlled trial. Pediatric Neurology 2021;123:10-20. doi: 10.1016/j.pediatrneurol.2021.05.014.
5. Berweck S, Bonikowski M, Kim H, Althaus M, Flatau-Baqué B, Mueller D, Banach MD. Placebo-Controlled Clinical Trial of IncobotulinumtoxinA for Sialorrhea in Children: SIPEXI. Neurology. 2021;97(14):e1425–36. doi: 10.1212/WNL.0000000000012573.
To cite this abstract in AMA style:
F. Heinen, M. Banach, E. Dabrowski, D. Gaebler-Spira, H. Chambers, S. Schroeder, T. Geister, M. Althaus, A. Hanschmann, S. Berweck. Safety of incobotulinumtoxinA in pediatrics: A pooled analysis [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/safety-of-incobotulinumtoxina-in-pediatrics-a-pooled-analysis/. Accessed February 6, 2026.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/safety-of-incobotulinumtoxina-in-pediatrics-a-pooled-analysis/