Category: Ataxia
Objective: To understand the scope of clinical research in cerebellar ataxia by performing a systematic review of controlled clinical trials over the past 50 years, focusing on patient demographics, therapies, the locations the trials were performed, and the funding mechanisms that supported them.
Background: Cerebellar ataxia is a relatively rare condition with multiple etiologies. While there is no approved treatment or cure for ataxia, advances in genetics and research into novel therapeutics indicate that treatment of ataxia is attainable.
Method: Clinical trials published in English were identified in scientific databases (Pubmed, SCOPUS, and Cochrane Central Register of Controlled Trials) from 1970 through December 2021, and additional non-published trials were identified in Clinical Trials.gov, and the World Health Organization (WHO) Home/International Clinical Trials Registry Platform (ICTRP). Trials were prospective, either single- or double-blinded (including blinded video assessments for neuromodulation trials), with a change in the severity of ataxia symptoms as the primary measure.
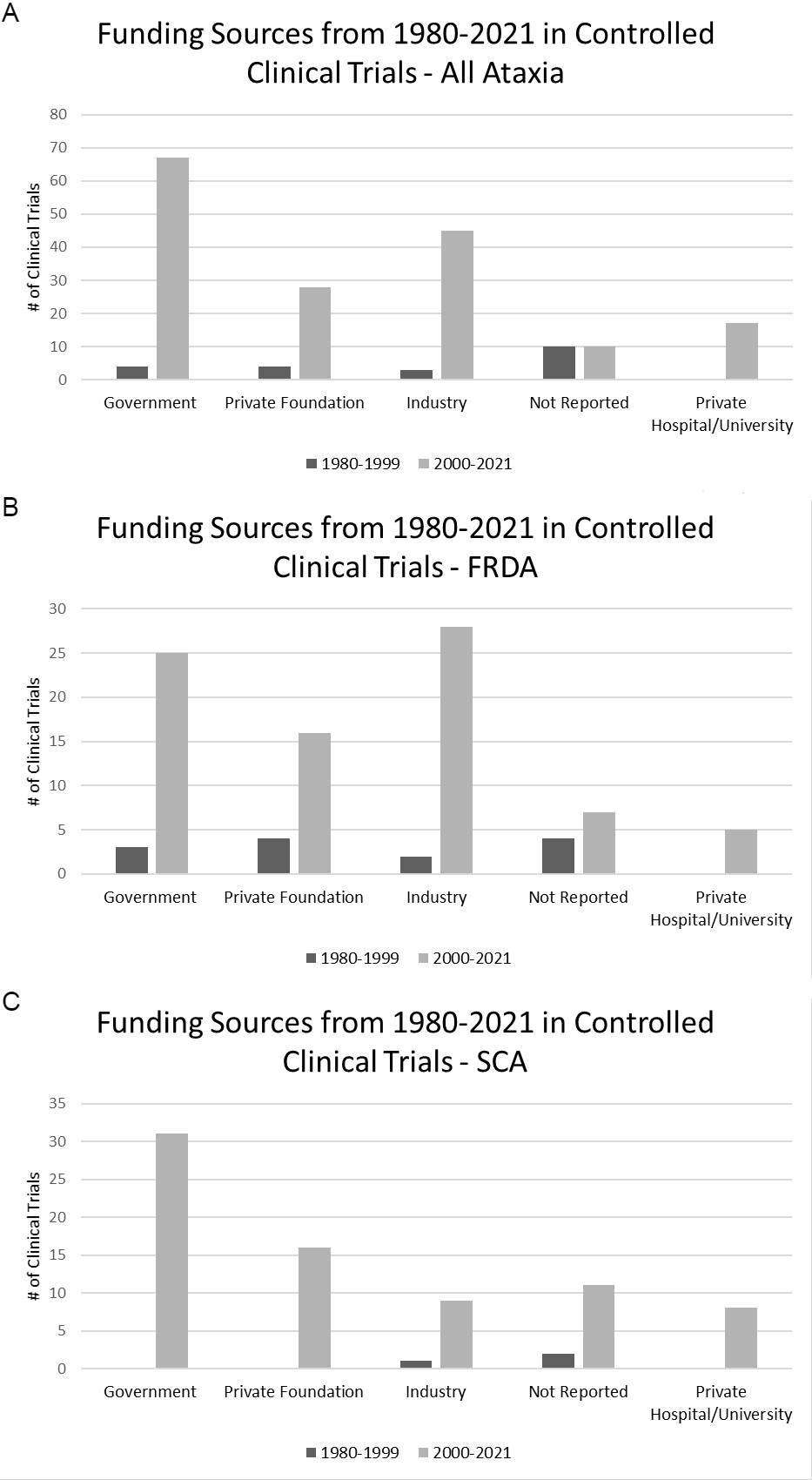
Results: Eighty-nine published clinical trials and fifteen actively recruiting trials met inclusion criteria. Of the published trials, 3,614 (3,625) patients were included, which consisted of 52% males in the 72 trials the reported gender. Only 8 trials reported race, comprising of 83% (81.4%) white, 14% (15%) Hispanic, and 3% African patients. Since 1980, 68.5% of trials were “pharmaceutical,” with the most common pharmaceutical being idebenone, a medication that was studied in Friedreich’s ataxia. There was significantly more funding in the last 20 years for cerebellar ataxia than the years from 1980-1999 (p = 0.0156) [figure 1]. Thirty-four percent of all published cerebellar ataxia clinical trials reported government funding, 21% had private foundation, 19% was funded by industry, 7% was funded by private hospitals/universities, and 19% of trials had “no funding reported”.
Conclusion: More clinical research is needed in all types of cerebellar ataxia. As research continues to pursue promising therapeutics, race should be reported in all clinical trials, with greater efforts towards diversity and outreach to underserved areas.
To cite this abstract in AMA style:
C. Kingsbury, S. Ghanekar, Y. Huang, T. Ashizawa, S. Kuo, C. Gooch, T. Zesiewicz. Therapies, Research Funding and Racial Diversity in Cerebellar Ataxia: A Systematic Review of the Literature [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/therapies-research-funding-and-racial-diversity-in-cerebellar-ataxia-a-systematic-review-of-the-literature/. Accessed July 1, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/therapies-research-funding-and-racial-diversity-in-cerebellar-ataxia-a-systematic-review-of-the-literature/