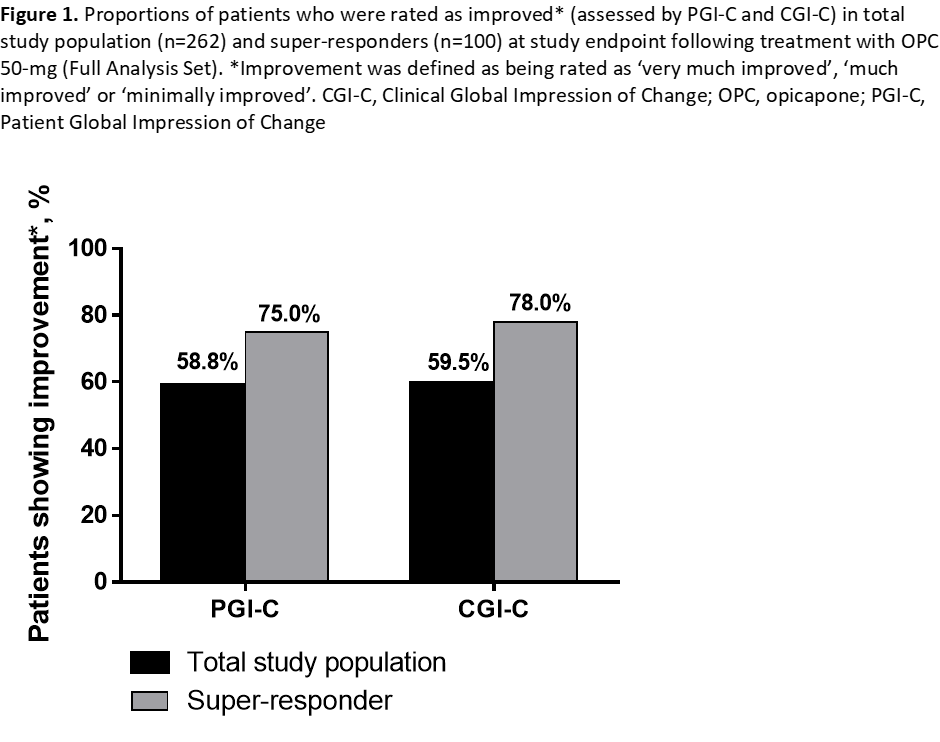Objective:
To evaluate the efficacy and safety of opicapone (OPC) in Parkinson’s disease (PD) patients who were considered ‘super-responders’ (>= 2 hours of OFF-time reduction or >= 2 hours of ON-time increase from baseline to double-blind endpoint).
Background: OPC, a once-daily catechol-O-methyltransferase inhibitor, proved to be effective in treating end-of-dose motor fluctuations in PD patients in two large multinational trials (BIPARK-I and II) [1,2].
Method: OPC 50-mg data from BIPARK I and II [1,2] were combined to evaluate the efficacy and safety of OPC 50-mg in patients who were considered super-responders. Efficacy was assessed by applying Patient and Clinician-Global Impression of Change (PGI-C and CGI-C). Safety was assessed by frequency of at least possibly related treatment-emergent adverse-events (TEAEs).
Results:
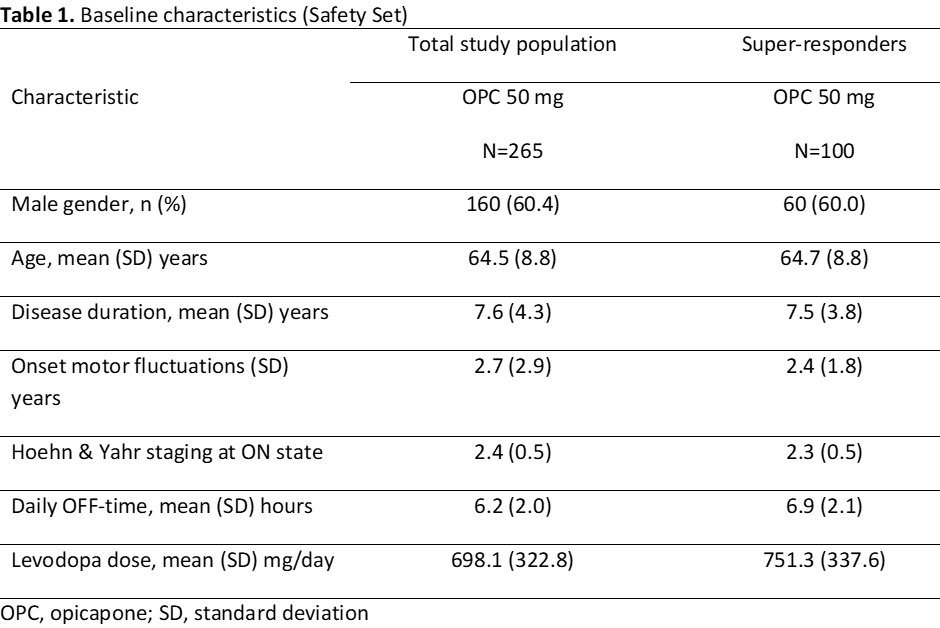
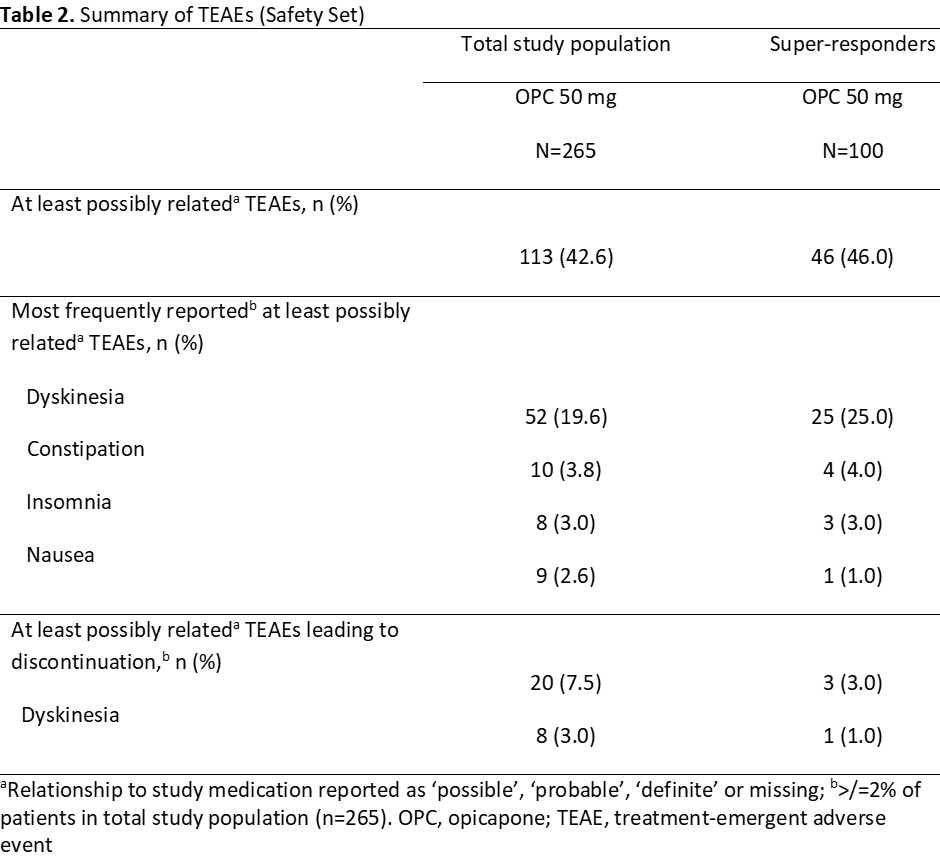
A total of 265 patients were treated with OPC 50-mg, of whom 100 were super-responders (Safety Set, Table 1). Super-responders had longer duration of daily OFF-time at baseline and were treated with a higher mean daily levodopa amount but had similar Hoehn and Yahr stage, disease duration and onset of motor fluctuations. The percentages of patients rated as showing improvement on both PGI-C and CGI-C were approximately 15% higher for super-responders than for total study population treated with OPC 50-mg (Figure 1). The frequency of at least possibly related TEAEs was similar, with higher dyskinesia rates in super-responders (most likely due to the higher mean daily levodopa at baseline) but low overall and dyskinesia-related discontinuations (Table 2).
Conclusion:
Super-responders to OPC 50-mg showed a high patient and clinician global impression of change and favourable tolerability.
References: 1. Ferreira et al., Lancet Neurol. 2016;15(2):154-165. 2. Lees et al., JAMA Neurol. 2017;74(2):197-206.
To cite this abstract in AMA style:
A. Antonini, W. Poewe, J. Ferreira, G. Ebersbach, H. Gama, J.F Rocha, D. Magalhães, P. Soares-da-Silva. Super-Responders to Opicapone Adjunct Treatment to Levodopa in Parkinson’s Disease Patients with Motor Fluctuations: Combined Post-Hoc Analysis of BIPARK-I and II [abstract]. Mov Disord. 2020; 35 (suppl 1). https://www.mdsabstracts.org/abstract/super-responders-to-opicapone-adjunct-treatment-to-levodopa-in-parkinsons-disease-patients-with-motor-fluctuations-combined-post-hoc-analysis-of-bipark-i-and-ii/. Accessed March 1, 2026.« Back to MDS Virtual Congress 2020
MDS Abstracts - https://www.mdsabstracts.org/abstract/super-responders-to-opicapone-adjunct-treatment-to-levodopa-in-parkinsons-disease-patients-with-motor-fluctuations-combined-post-hoc-analysis-of-bipark-i-and-ii/