Category: Parkinson’s Disease: Clinical Trials
Objective: The primary objective was to determine the comparative bioavailability between a marketed and a new levodopa dry powder inhaler (DPI) with an enhanced usability profile. The most important secondary objective was to determine the safety and tolerability of this investigational levodopa DPI up to and including a 135 mg dose.
Background: Many PD patients experience OFF episodes when their disease progresses. OFF episodes are very debilitating and require medication with a predictable and fast onset of effect. This unmet medical need was addressed by apomorphine-based products and a marketed levodopa DPI, but these products still have limitations for the patient. This new levodopa DPI is a pre-filled, ready-to-use, single-use DPI that offers PD patients excellent ease-of-use. Previous studies in PD patients have shown its rapid onset of action, ease-of-use during OFF episodes, fast efficacy (<20 minutes) and tolerability. [1][2]
Method: The study concerns an open-label, randomized, crossover, pharmacokinetic bioavailability study in which two levodopa DPIs are compared in 26 healthy adult subjects. Investigated doses were 45, 90 and 135 mg for the new levodopa DPI and 84 mg for the marketed DPI, combined with carbidopa. Investigated PK and safety endpoints were Cmax, Tmax, AUC0-4, AUC0-t, AUCinf, t½, adverse events, vital signs and clinical/laboratory examination. Most important objectives were to determine the comparative bioavailability, and the safety and tolerability of the new DPI.
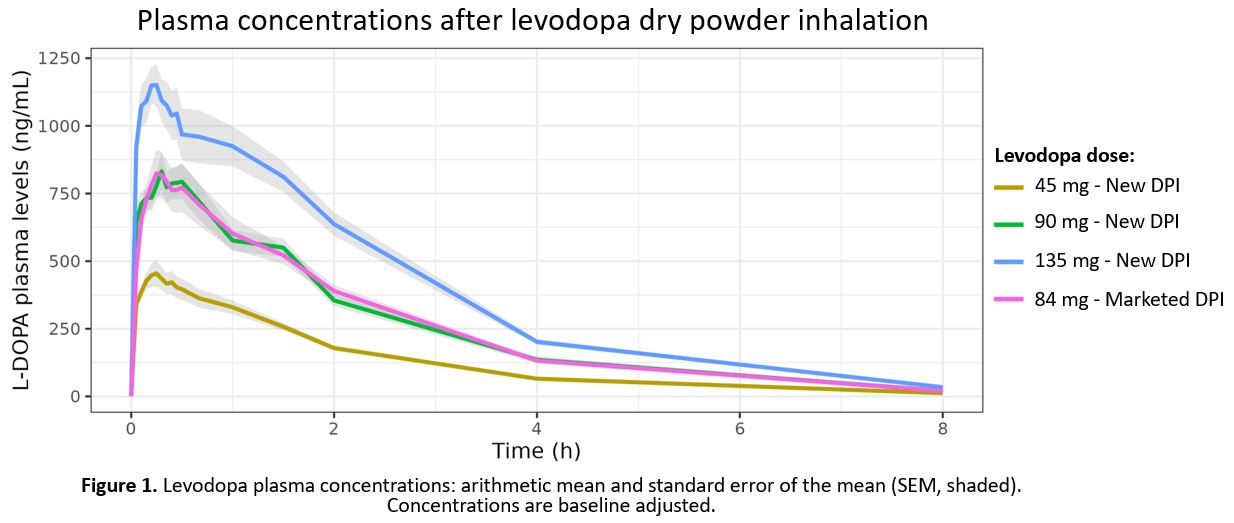
Results: ~2000 plasma samples were analyzed by LC-MS/MS. The results [figure1] show a linear increase in concentration and the shape of the curves is very similar, but absorption during the first 9 minutes is somewhat faster with the new DPI. Only one case of levodopa-related diarrhea was reported as adverse event and no reports of cough for the new DPI.
Conclusion: The investigational levodopa DPI is safe and very well tolerated. Levodopa absorption from both DPIs is comparable, thereby, fulfilling the bioequivalence criteria, albeit with improved usability. Because the new levodopa DPI is so easy to use and systemic absorption is very fast, it will be a valuable asset to offer fast and reliable relief from debilitating OFF episodes.
Baseline adjusted levodopa plasma concentrations.
References: [1] Luinstra et al. (2019), Learning from Parkinson’s patients: Usability of the Cyclops dry powder inhaler, International Journal of Pharmaceutics, p. 1-5, vol. 567
[2] Luinstra et al. (2019), Pharmacokinetics and tolerability of inhaled levodopa from a new dry-powder inhaler in patients with Parkinson’s disease, Therapeutic Advances in Chronic Disease, 204062231985761, vol. 10
To cite this abstract in AMA style:
T. van Laar, F. Grasmeijer, M. Hoppentocht. A Comparative Bioavailability Study between a Marketed Capsule-based Levodopa Dry Powder Inhaler and a New Pre-filled Levodopa Dry Powder Inhaler [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/a-comparative-bioavailability-study-between-a-marketed-capsule-based-levodopa-dry-powder-inhaler-and-a-new-pre-filled-levodopa-dry-powder-inhaler/. Accessed July 5, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-comparative-bioavailability-study-between-a-marketed-capsule-based-levodopa-dry-powder-inhaler-and-a-new-pre-filled-levodopa-dry-powder-inhaler/