Category: Parkinson’s Disease: Clinical Trials
Objective: To evaluate the safety and effects of add-on safinamide on apathy in patients with Parkinson’s disease (PD).
Background: Apathy is highly prevalent and disabling in PD. Safinamide, a reversible monoamine oxidase-B inhibitor and modulator of abnormal glutamate release, suggested to decrease non-motor system burden in PD, has not been formally tested for apathy in PD.
Method: Prospective, 24-week, two-site, randomized, double-blind, placebo-controlled, parallel group, investigator initiated exploratory study (EudraCT 2017-003254-17) in non-demented PD on stable dopaminergic therapy randomized 1:1 to adjunct safinamide (50mg/day for 2 weeks and 100mg/day for 22 weeks) or placebo.
The primary endpoint was mean change from baseline to week 24 on the Apathy Scale (AS) total score. Secondary endpoints included changes in cognition, activities of daily living, motor scores, impression of change, safety and tolerability measures.
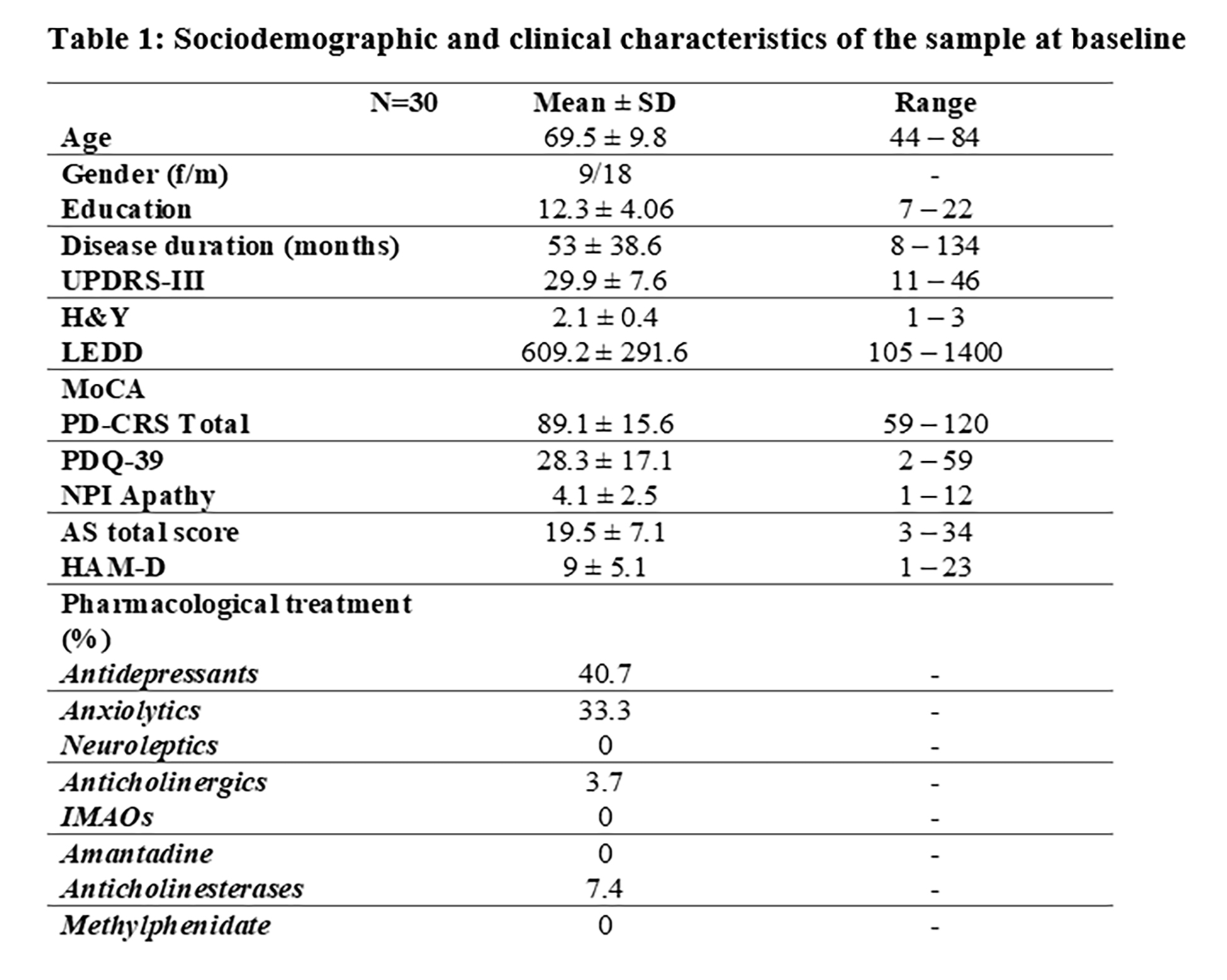
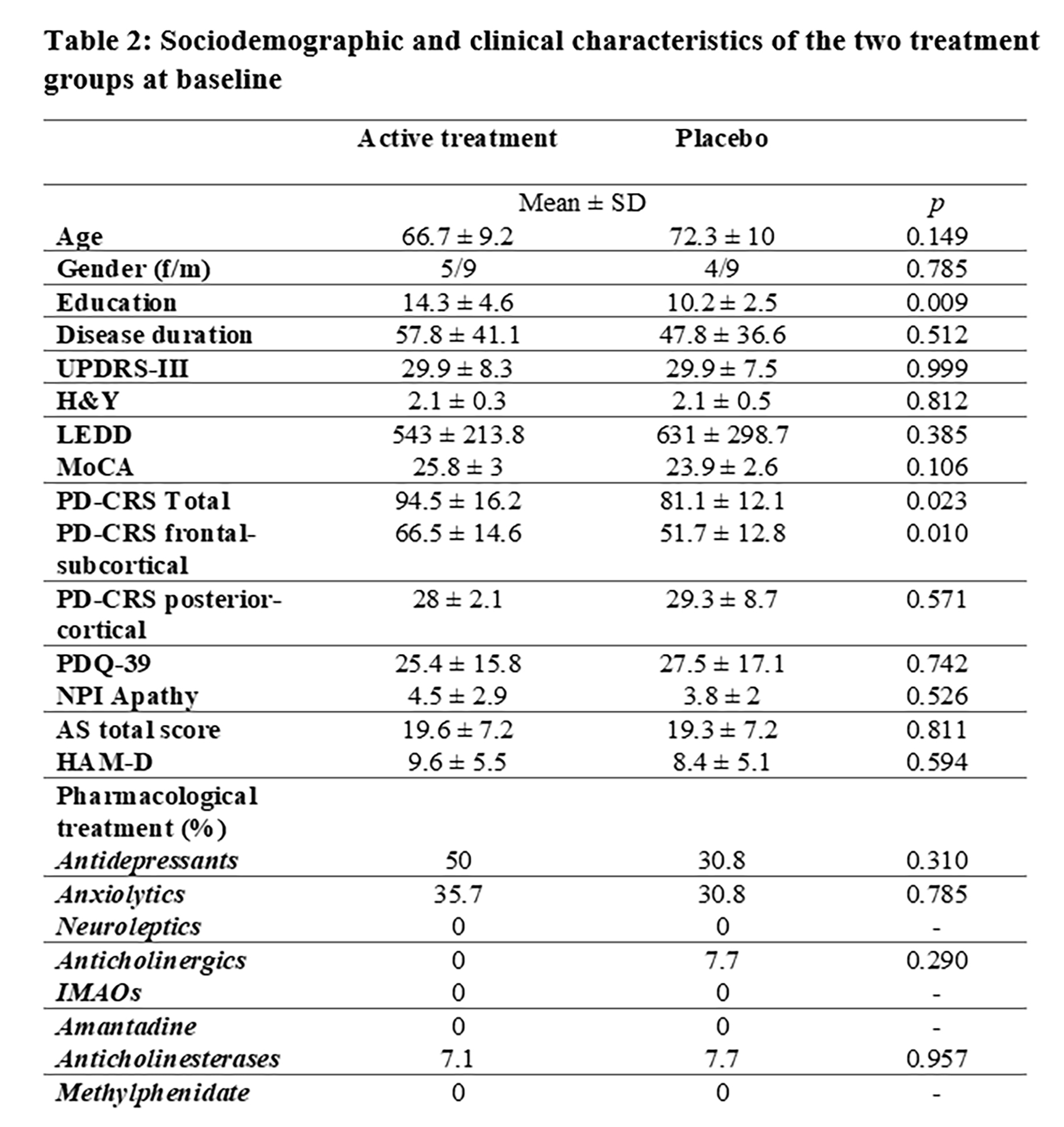
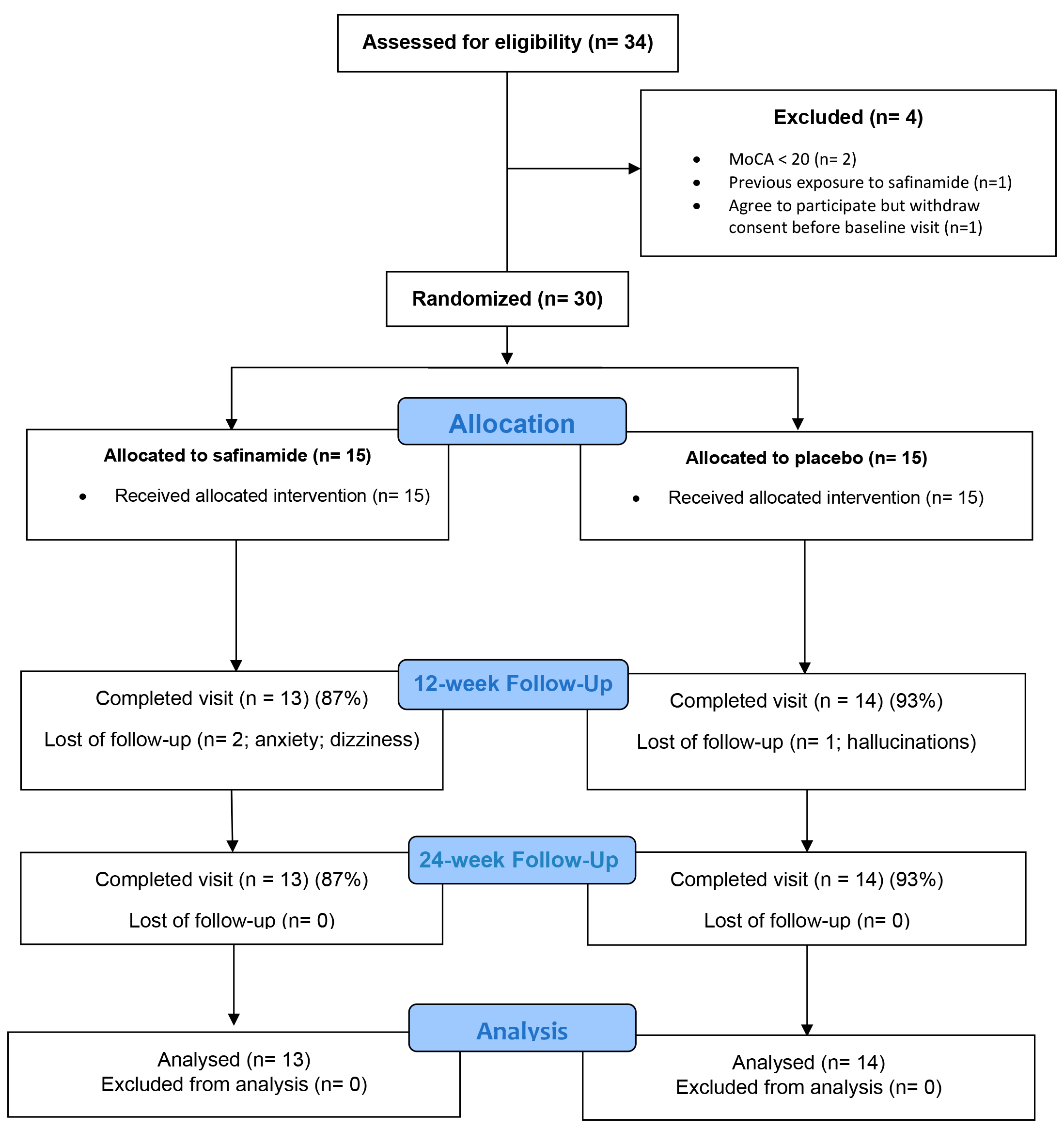
Results: From 34 screened people with PD (PwP), 30 eligible subjects (Figure 1, table 1) (80% showing clinically significant apathetic symptoms according to the AS) were enrolled, and included in the intention-to-treat analysis (active treatment=15; placebo=15; table 2).
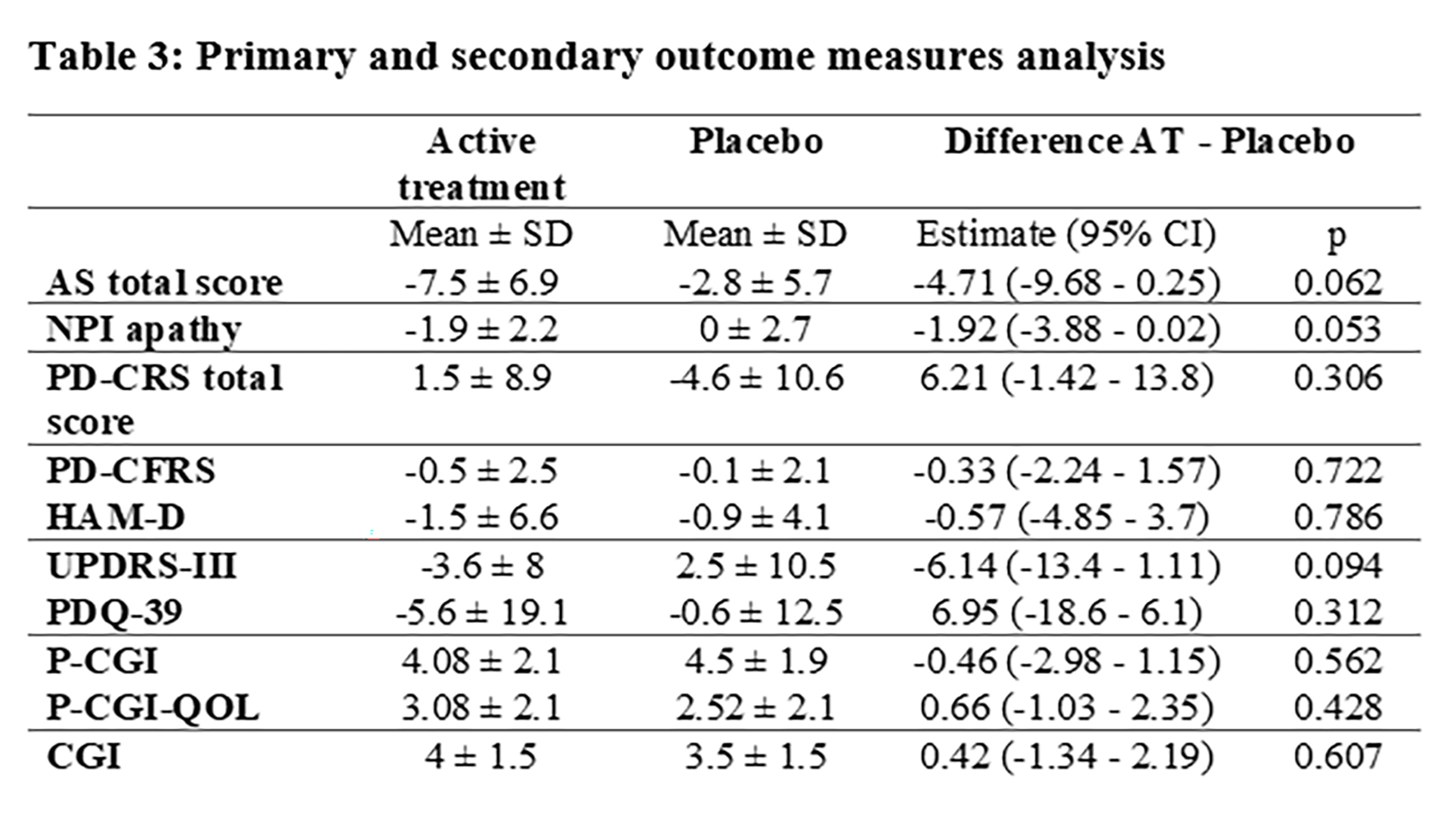
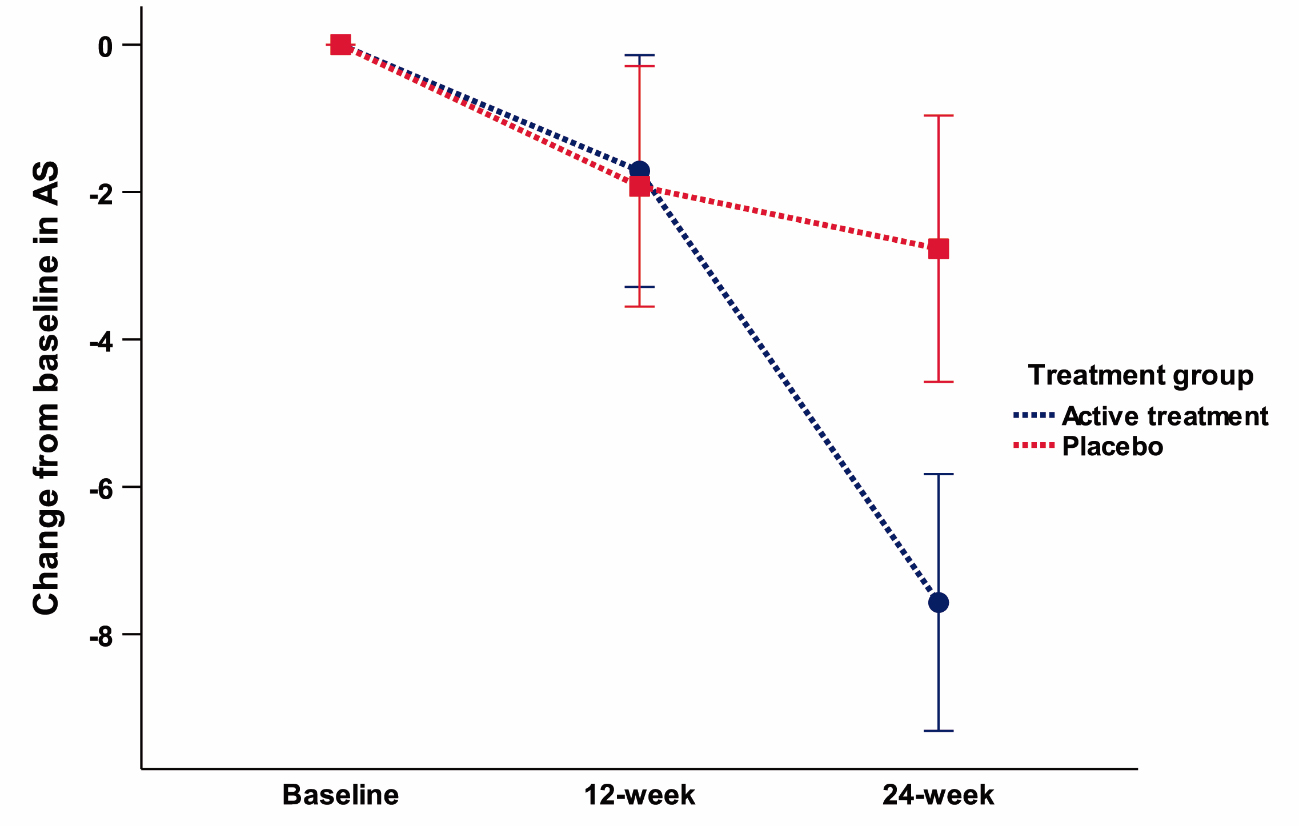
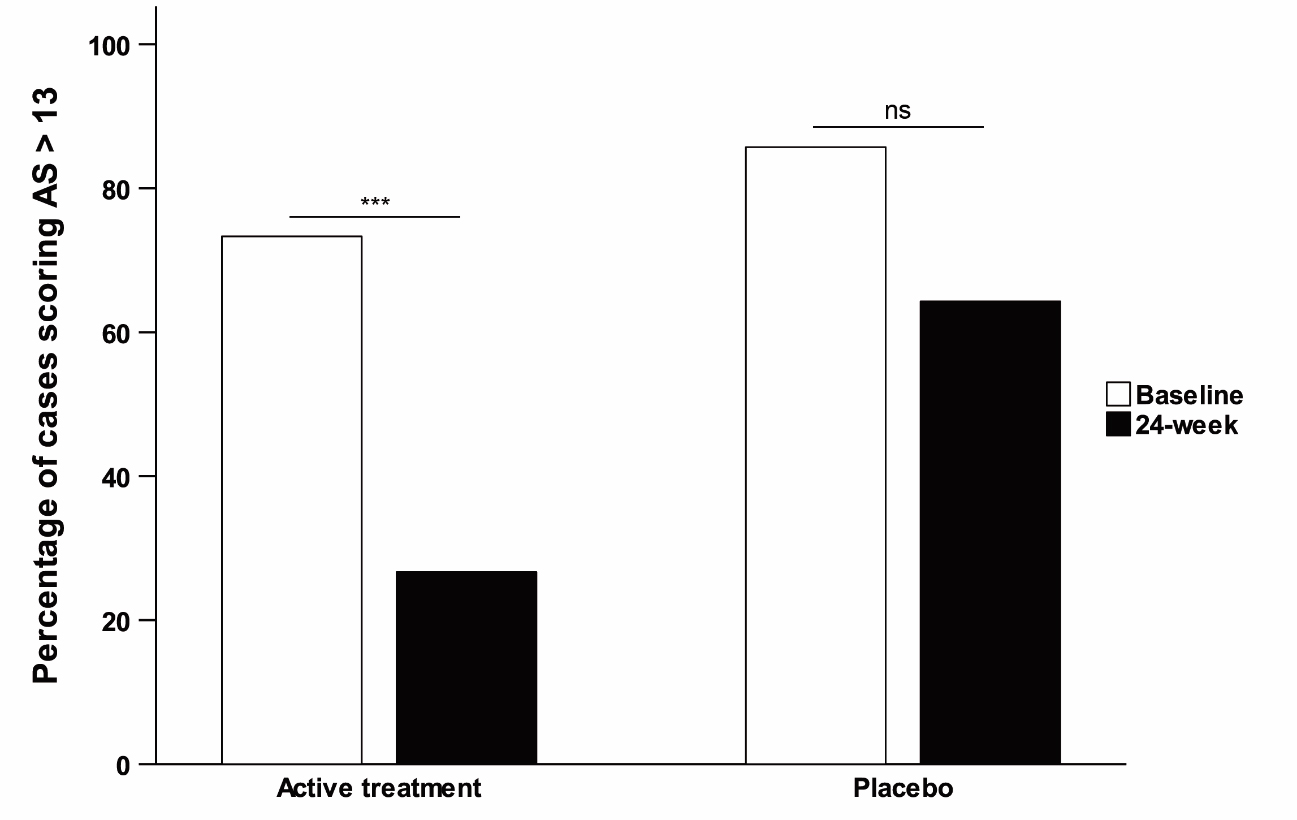
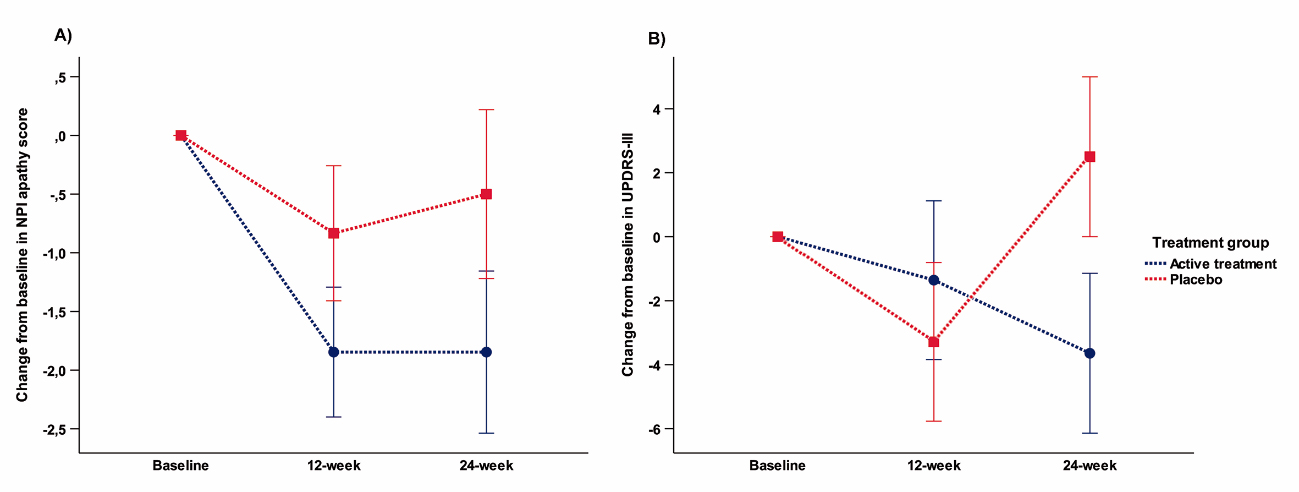
Adverse events were few and only mild in both treatment groups. Change in AS (ANOVA) showed a trend to significance [p=0.059] mediated by a more marked decrease on AS score with safinamide (-7.5±6.9) than with placebo (-2.8±5.7). Post-hoc analysis (paired t-test) showed a significant positive change in the AS score between 12-week and 24-week [p=0.001] only in the active group (figure 2,3). No significant or trend changes were found for any of the secondary outcome variables. Post-hoc t-test comparison showed a trend to significant improvement [t(30) = -2.06; p = 0.053] in the NPI total score for apathy (frequency x severity) at 24-week mediated by a mean change from baseline of -1.9 ± 2.2 points in the safinamide group compared to 0 ± 2.7 in the placebo group (figure 4). No effects were found for the other neuropsychiatric symptoms covered with the NPI.
Conclusion: Safinamide was safe and well tolerated, but failed to provide evidence of improved apathy. Positive signals on post-hoc analysis showing delayed improvement of AS possibly related to beginning of anti-glutamatergic activity warrants a larger RCT.
To cite this abstract in AMA style:
J. Kulisevsky, S. Martinez-Horta, A. Campolongo, B. Pascual-Sedano, J. Marin-Lahoz, H. Bejr-Kasem, A. Horta-Barba, A. Puig-Davi, J. Pagonabarraga, I. Aracil-Bolaños, J. Rodriguez-Antiguedad. A randomized clinical trial (RCT) to evaluate the effects of safinamide on apathetic non-demented Parkinson’s disease patients [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/a-randomized-clinical-trial-rct-to-evaluate-the-effects-of-safinamide-on-apathetic-non-demented-parkinsons-disease-patients/. Accessed June 30, 2025.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/a-randomized-clinical-trial-rct-to-evaluate-the-effects-of-safinamide-on-apathetic-non-demented-parkinsons-disease-patients/