Objective: To evaluate 12-month adherence and persistence and identify factors associated with persistence among Parkinson’s disease (PD) patients initiating istradefylline, the first non-dopaminergic adjunct targeting the adenosine A2A pathway.
Background: Adherence and persistence to medication are associated with better patient outcomes. There are no real-world data on istradefylline adherence and persistence in the US.
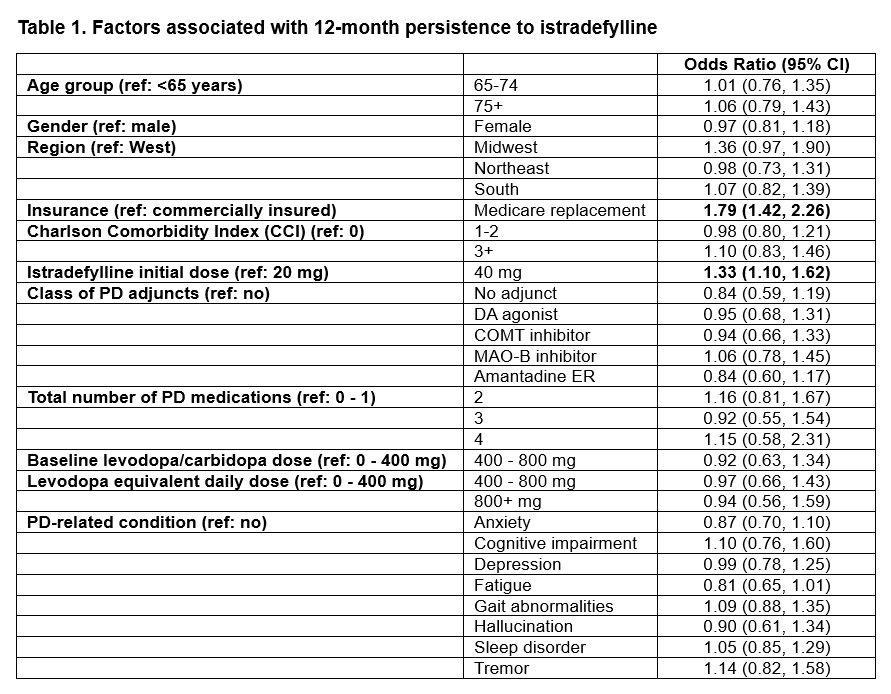
Method: A retrospective cohort study was conducted using IQVIA longitudinal prescription (LRx) and medical (Dx) claims data. Adult patients initiating istradefylline in 2019-2023 with ≥ 1 LRx and ≥ 1 Dx claims every 6 months in the 12-month baseline and follow-up period were included. Persistence was measured as the duration of time a patient continued to fill istradefylline prescriptions with a 60-day grace period. Adherence was measured by Proportion of Days Covered (PDC), the percentage of days covered by refills of istradefylline over 12 months. Multivariate logistic regression was used to assess the association of 12-month persistence with patient demographics, istradefylline initial dose, class of PD adjunct medications, total number of PD medications, baseline levodopa/carbidopa dose, levodopa equivalent daily dose, and PD-related conditions.
Results: This study included 2,045 patients (mean age: 72.9 years; female: 39.4%). Overall, 76.0% of them were covered by a Medicare replacement plan and 24.0% were commercially insured. A majority (67.3%) initiated istradefylline at 20 mg (vs. 40 mg). However, 27.5% of those patients were titrated up to 40 mg in 12 months. Only 6.7% initiating treatment at 40 mg were titrated down to 20 mg. Median persistence was 276 days (IQR: 90, 365 days) and PDC was 74.0% (IQR: 24.7%, 98.1%) in 12 months. Patients initiating treatment at 40 mg were more likely to be persistent to treatment (OR: 1.33 [95% CI: 1.10, 1.62]). Medicare replacement was also independently associated with persistence (OR: 1.79 [95% CI: 1.42, 2.26]). Persistence (median: 365 days; IQR: 90, 365 days) and PDC (median: 82.2%; IQR: 24.7%, 98.6%) were significantly higher in Medicare replacement vs. commercially insured patients (p<0.0001).
Conclusion: Patients initiating istradefylline at 40 mg and those covered by Medicare replacement were more likely to have a higher adherence and longer persistence to istradefylline.
Table 1
To cite this abstract in AMA style:
R. Joseph, J. Qian, H. Cummings, Y. Chen, S. Tewari, M. Soileau. Adherence and Persistence Among Patients with Parkinson’s Disease Initiating Istradefylline: 12-Month Follow-Up from US Real-World Data [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/adherence-and-persistence-among-patients-with-parkinsons-disease-initiating-istradefylline-12-month-follow-up-from-us-real-world-data/. Accessed July 5, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/adherence-and-persistence-among-patients-with-parkinsons-disease-initiating-istradefylline-12-month-follow-up-from-us-real-world-data/