Category: Huntington's Disease
Objective: To investigate the presence of beta-amyloid pathology in Huntington’s disease (HD) and its potential impact on patients’ cognitive status.
Background: Recent evidence indicates that beyond the accumulation of mutant Huntingtin protein (mHTT), tau and other proteinopathies are related to the clinical phenotypes of HD. Postmortem studies have suggested the presence of beta-amyloid and phosphorylated tau co-pathology in the HD brains [1], but reports based on CSF quantification have shown conflicting data regarding the levels of beta-amyloid peptides (A𝛃42 and A𝛃40) and amyloid precursor protein (APP) [2,3]. Notably, no studies have explored the possible association between beta-amyloid pathology and cognitive status in HD.
Method: A descriptive cross-sectional study was conducted on genetically confirmed HD patients and age-matched healthy controls. CSF levels of A𝛃42, A𝛃40, A𝛃42/A𝛃40 ratio, neurofilament light chain (NfL), total tau protein (t-Tau), and phosphorylated tau (pTau231) were quantified using the Simoa platform. Clinical and motor assessments were performed to evaluate disease severity. A comprehensive neuropsychological assessment was conducted focusing on global cognition using the Parkinson’s Disease-Cognitive Rating Scale (PD-CRS), and on standardized assessments covering episodic memory, language, executive function and attention, processing speed, and visuoperceptive/visuospatial processes.
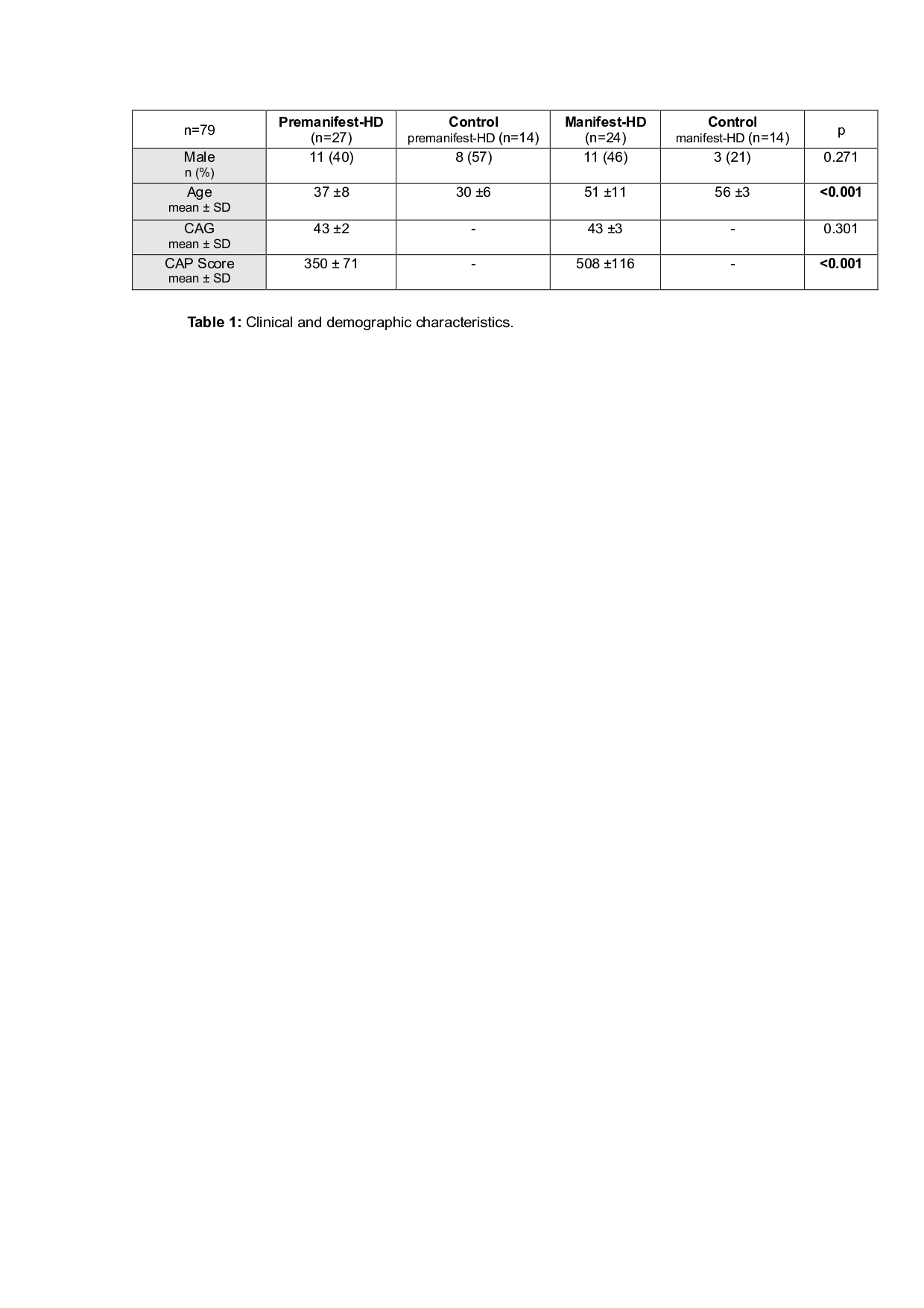
Results: Seventy-nine individuals were included: 51 HD patients (27 premanifest, 24 manifest) and 28 controls [Table 1].
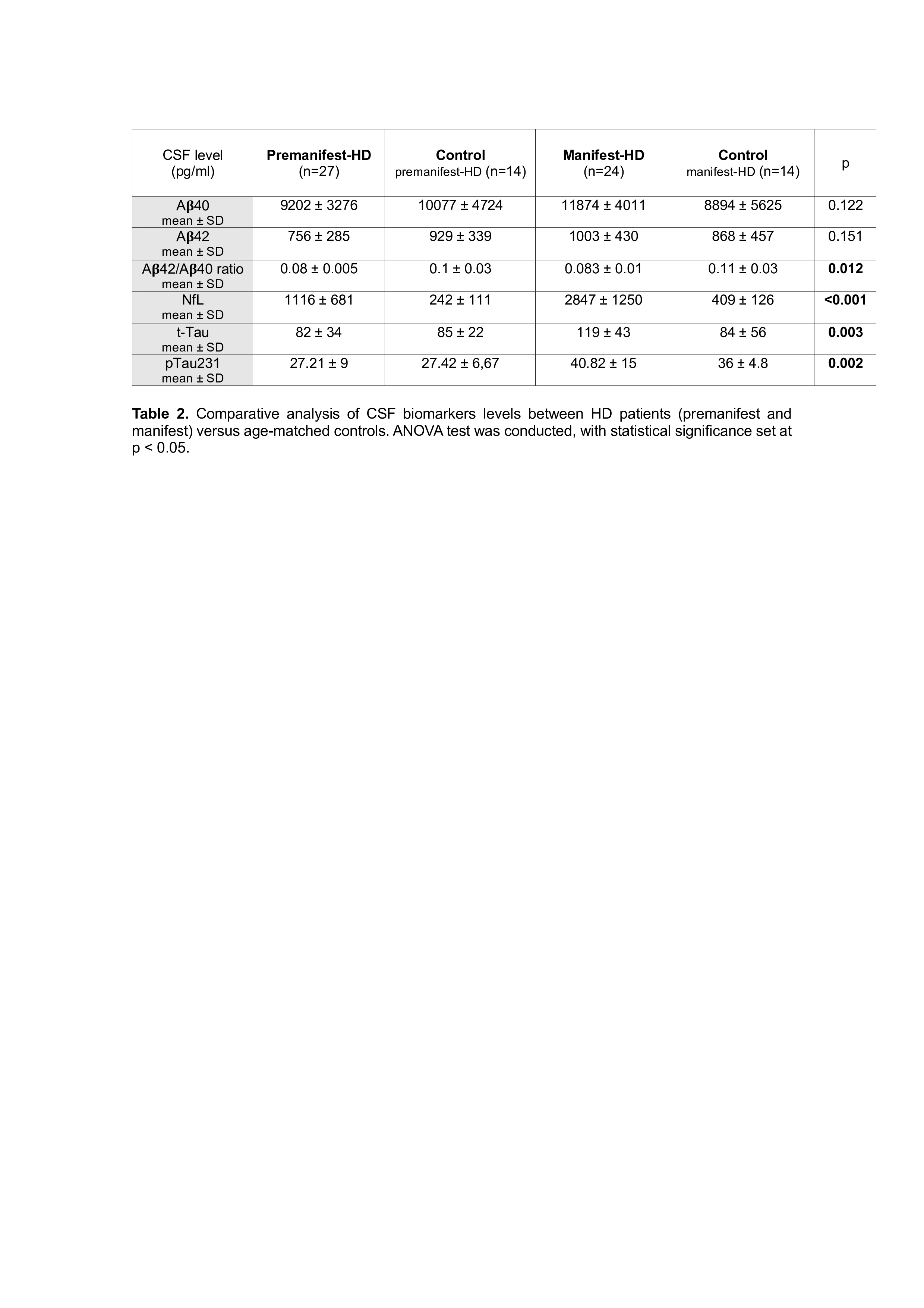
While no differences were observed in A𝛃42 or A𝛃40 levels between groups, a statistically significant difference was found in the A𝛃42/A𝛃40 ratio (p=0.012) [Table 2]. However, the magnitude of the effect was small, and none of the patients exhibited pathological levels of A𝛃 peptides or the A𝛃42/A𝛃40 ratio (according to the limits established in our laboratory).
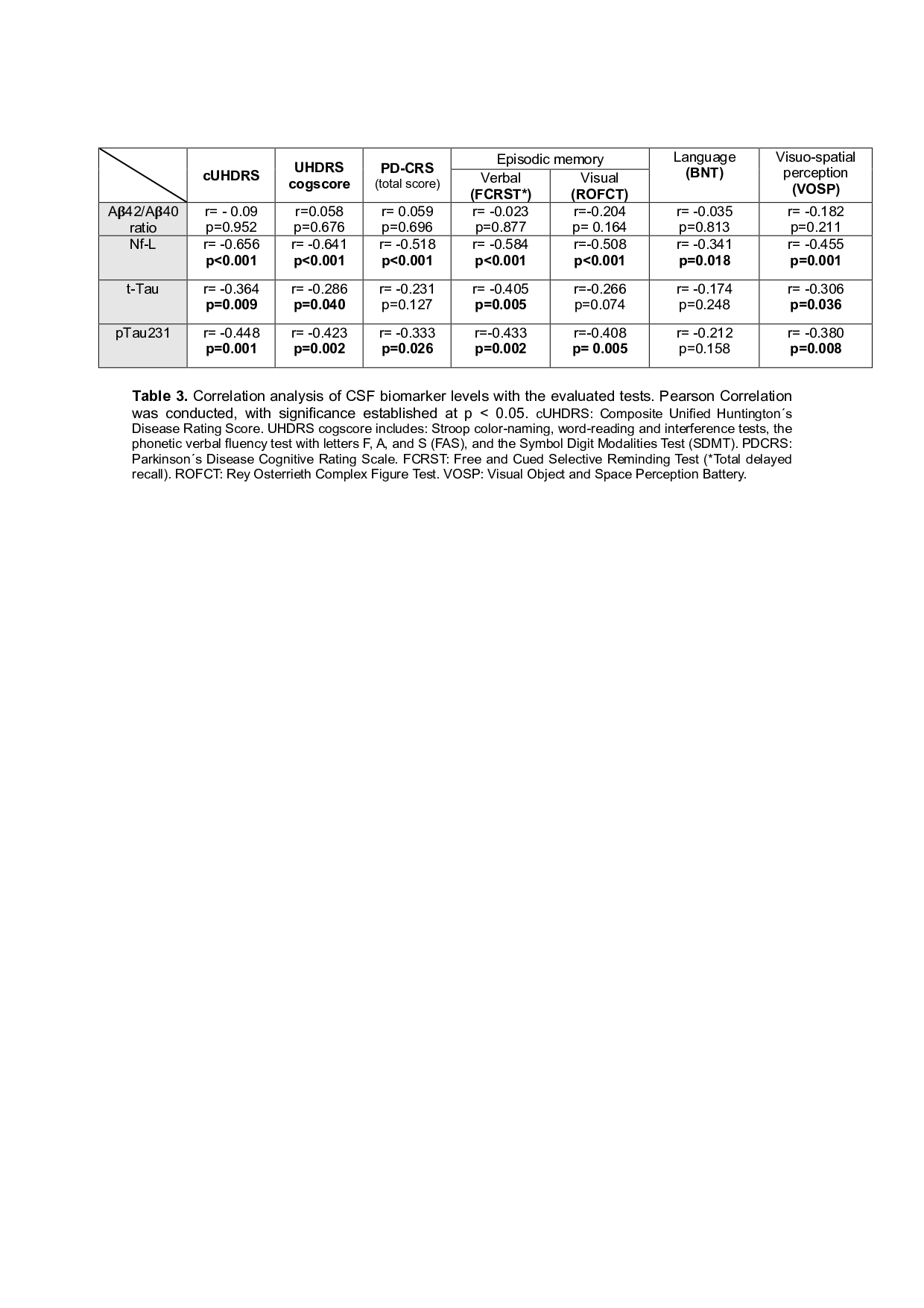
The A𝛃42/A𝛃40 ratio and A𝛃42 CSF levels showed no association with cognitive status. Conversely, NfL, t-Tau, and p231Tau CSF levels were negatively associated with cognition in several tests [Table 3].
Conclusion: Our findings suggest that, contrary to tau, beta-amyloid pathology does not significantly contribute to basal cognitive status in HD.
Table 1
Table 2
Table 3
References: 1. Davis MY, Keene CD, Jayadev S, Bird T. The co-occurrence of Alzheimer’s disease and Huntington’s disease: a neuropathological study of 15 elderly Huntington’s disease subjects. J Huntingtons Dis. 2014;3(2):209-17. doi: 10.3233/JHD-140111.
2. Lowe AJ, Sjödin S, Rodrigues FB, Byrne LM, Blennow K, Tortelli R, et al. Cerebrospinal fluid endo-lysosomal proteins as potential biomarkers for Huntington’s disease. PLoS ONE. 2020;15(8): e0233820. doi: 10.1371/journal.pone.0233820.
3. Ye LQ, Li XY, Zhang YB, Cheng HR, Ma Y, Chen DF, Tao QQ, Li HL, Wu ZY. The discriminative capacity of CSF AB-amyloid 42 and Tau in neurodegenerative diseases in the Chinese population. J Neurol Sci. 2020 May 15; 412:116756. doi: 10.1016/j.jns.2020.116756.
To cite this abstract in AMA style:
G. Olmedo Saura, J. Perez Perez, S. Martinez-Horta, A. Puig-Davi, A. Horta-Barba, A. Vazquez-Oliver, A. Campolongo, E. Rivas-Asensio, N. Salvat-Rovira, D. Alcolea, J. Pagonabarraga, J. Kulisevsky. Amyloid Pathology and its association with Cognitive Status in Huntington’s Disease [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/amyloid-pathology-and-its-association-with-cognitive-status-in-huntingtons-disease/. Accessed July 10, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/amyloid-pathology-and-its-association-with-cognitive-status-in-huntingtons-disease/