Category: Parkinson's Disease: Non-Motor Symptoms
Objective: We aim to use objective polysomnography to analyze the relationship between dyskinesia and sleep structure in patients with Parkinson’s disease.
Background: Levodopa is an effective drug for the Parkinson’s disease. At present, it is known that there is a certain relationship between dyskinesia and sleep.Previous studies have confirmed that Parkinson’s disease (PD) patients with dyskinesia have worse subjective sleep quality, but there are few related clinical studies.
Method: 122 patients with Parkinson’s disease from the Second Affiliated Hospital of Soochow University were included. According to the score of the 32nd item of UPDRS IV, the patients were divided into 2 groups: dyskinesia group (DYS, n=27), without dyskinesia group (non-DYS, n=95). The demographic information was collected. Polysomnography was used to record sleep information.85 patients with complete polysomnography (PSG) EEG data were divided into DYS group (n=23) and non-DYS group (n=62). We measured the demographic characteristics of the two groups and the differences in EEG Spectral density in each frequency range.
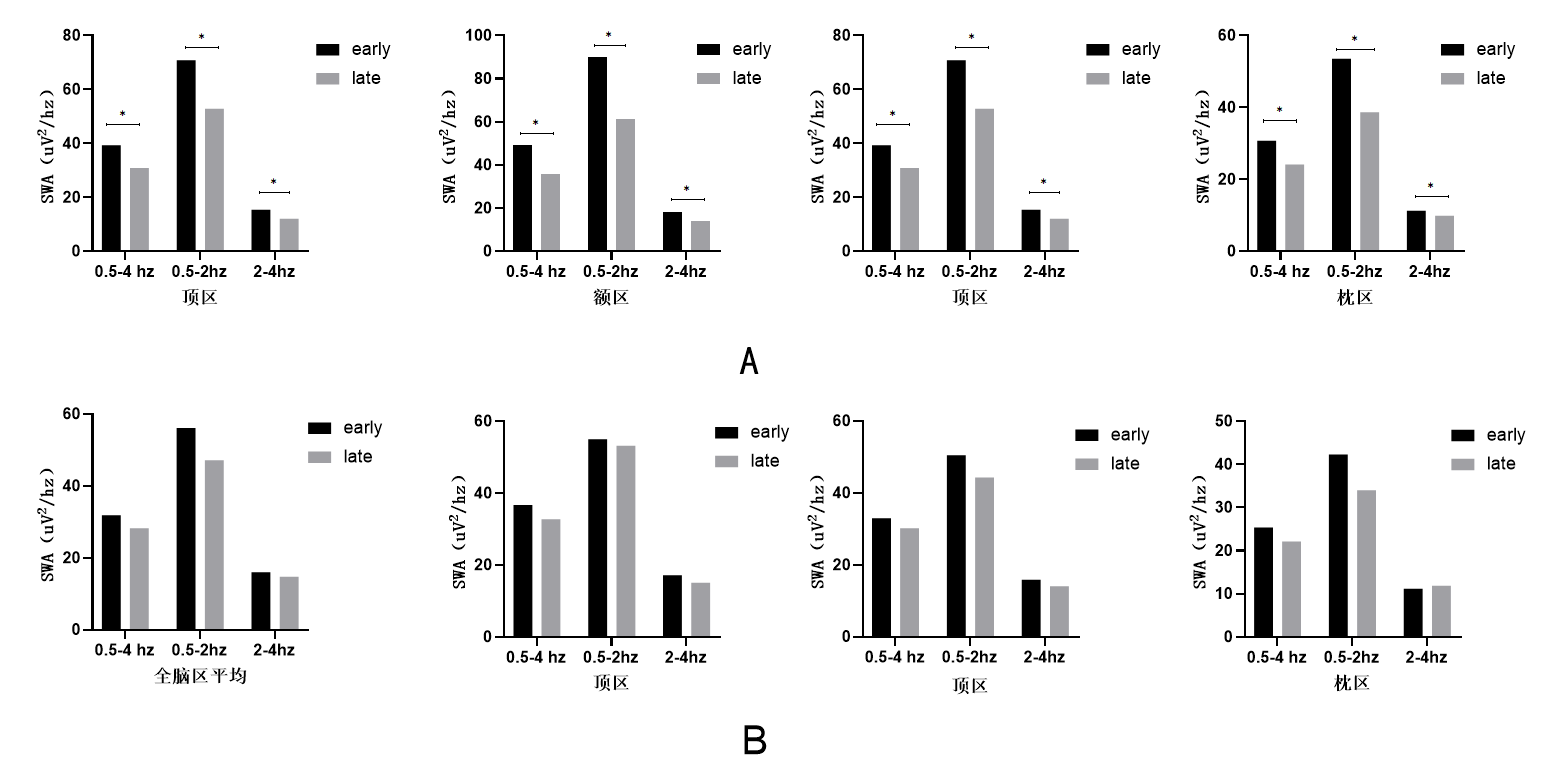
Results: Compared with the non-DYS group, the DYS group had more women, longer disease duration, higher levodopa equivalent dose, and a significantly higher proportion of NREM sleep.After adjusting for confounding factors, the proportion of NREM sleep is an independent risk factor for the occurrence of dyskinesia (OR 1.141, 95% CI 1.024-1.273, p =0.017). In addition, compared with patients in the non-DYS group, patients in the DYS group had lower frontal slow-wave EEG spectral density during NREM sleep all night. At the same time, it was also observed that patients in the DYS group had no significant SWA spectral density downscale during NREM sleep. After adjusting for confounding factors, the logistic regression analysis showed that low-frequency (0.5-2hz) SWA changes in the frontal area were independently associated with dyskinesia (OR 0.58; 95% CI 0.376-0.894, p =0.014).
Conclusion: This study verifies the conclusions of previous studies, revealing that there is disorder in sleep structure and EEG in PD patients with dyskinesia. It is important to pay attention to the sleep quality of patients with Parkinson’s disease with dyskinesia, especially for the patients who had higher proportion of NREM and whose frontal lobe channel SWA does not change significantly during NREM sleep throughout the night.
References: [1] Hirsch E C, Jenner P, Przedborski S. Pathogenesis of Parkinson’s disease [J]. Mov Disord, 2013, 28(1): 24-30. [2] LeWitt P A. Levodopa therapy for Parkinson’s disease: Pharmacokinetics and pharmacodynamics [J]. Mov Disord, 2015, 30(1): 64-72. [3] Tambasco N, Romoli M, Calabresi P. Levodopa in Parkinson’s Disease: Current Status and Future Developments [J]. Curr Neuropharmacol, 2018, 16(8): 1239-1252. [4] Fabbrini G, Brotchie J M, Grandas F, et al. Levodopa-induced dyskinesias [J]. Mov Disord, 2007, 22(10): 1379-1389; [5] Bastide M F, Meissner W G, Picconi B, et al. Pathophysiology of L-dopa-induced motor and non-motor complications in Parkinson’s disease [J]. Prog Neurobiol, 2015, 132(96-168). [6] Rajan R, Popa T, Quartarone A, et al. Cortical plasticity and levodopa-induced dyskinesias in Parkinson’s disease: Connecting the dots in a multicomponent network [J]. Clin Neurophysiol, 2017, 128(6): 992-999. [7] Kishore A, Popa T, Balachandran A, et al. Cerebellar sensory processing alterations impact motor cortical plasticity in Parkinson’s disease: clues from dyskinetic patients [J]. Cereb Cortex, 2014, 24(8): 2055-2067. [8] Shen Y, Liu C F. Sleep Disorders in Parkinson’s Disease: Present Status and Future Prospects [J]. Chin Med J (Engl), 2018, 131(8): 883-885. [9] Monti J M, Monti D. The involvement of dopamine in the modulation of sleep and waking [J]. Sleep Med Rev, 2007, 11(2): 113-133. [10] Tononi G, Cirelli C. Sleep and the price of plasticity: from synaptic and cellular homeostasis to memory consolidation and integration [J]. Neuron, 2014, 81(1): 12-34. [11] Vyazovskiy V V, Riedner B A, Cirelli C, et al. Sleep homeostasis and cortical synchronization: II. A local field potential study of sleep slow waves in the rat [J]. Sleep, 2007, 30(12): 1631-1642. [12] Galati S, Salvade A, Pace M, et al. Evidence of an association between sleep and levodopa-induced dyskinesia in an animal model of Parkinson’s disease [J]. Neurobiol Aging, 2015, 36(1577-1589). [13] van Gilst M M, Bloem B R, Overeem S. “Sleep benefit” in Parkinson’s disease: a systematic review [J]. Parkinsonism Relat Disord, 2013, 19(7): 654-659. [14] Mao C J, Yang Y P, Chen J P, et al. Poor nighttime sleep is positively associated with dyskinesia in Parkinson’s disease patients [J]. Parkinsonism Relat Disord, 2018, 48(68-73). [15] Amato N, Manconi M, Moller J C, et al. Levodopa-induced dyskinesia in Parkinson disease: Sleep matters [J]. Ann Neurol, 2018, 84(6): 905-917. [16] Tomlinson C L, Stowe R, Patel S, et al. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease [J]. Mov Disord, 2010, 25(15): 2649-2653. [17] Lunardi G, Galati S, Tropepi D, et al. Correlation between changes in CSF dopamine turnover and development of dyskinesia in Parkinson’s disease [J]. Parkinsonism Relat Disord, 2009, 15(5): 383-389. [18] Walker N A, Sunderram J, Zhang P, et al. Clinical utility of the Epworth sleepiness scale [J]. Sleep Breath, 2020, 24(4): 1759-1765. [19] Buysse D J, Reynolds C F, 3rd, Monk T H, et al. The Pittsburgh Sleep Quality I ndex: a new instrument for psychiatric practice and research [J]. Psychiatry Res, 1989, 28(2): 193-213. [20] Hamilton M. The assessment of anxiety states by rating [J]. Br J Med Psychol, 1959, 32(1): 50-55. [21] Hamilton M. A rating scale for depression [J]. J Neurol Neurosurg Psychiatry, 1960, 23(1): 56-62.
To cite this abstract in AMA style:
YM. Wang, CJ. Mao. Analysis of Sleep Structure and EEG Characteristics of Patients with Dyskinesia in Parkinson’s Disease [abstract]. Mov Disord. 2021; 36 (suppl 1). https://www.mdsabstracts.org/abstract/analysis-of-sleep-structure-and-eeg-characteristics-of-patients-with-dyskinesia-in-parkinsons-disease/. Accessed July 3, 2025.« Back to MDS Virtual Congress 2021
MDS Abstracts - https://www.mdsabstracts.org/abstract/analysis-of-sleep-structure-and-eeg-characteristics-of-patients-with-dyskinesia-in-parkinsons-disease/