Category: Parkinson's Disease: Neuroimaging
Objective: To assess white matter (WM) microstructural integrity changes in pre-clinical models of Parkinson’s disease (PD) with levodopa-induced dyskinesia (LID).
Background: PD is a neurodegenerative disease that leads to significant disability and morbidity [1]. Levodopa (L-DOPA) is one of the primary pharmacological treatments for PD to alleviate the classic motor symptoms. However, long-term efficacy of L-DOPA is limited, and up to 90% of patients develop LID within ten years [2]. Increased fractional anisotropy (FA) from DTI has previously been reported in both PD patients with LID compared to those without LID [3] and in the 6-hydroxydopamine (6-OHDA) rat model of parkinsonism [4]. Here, we assessed longitudinal WM changes using FA between sham, 6-OHDA with LID, and 6-OHDA without LID rats over a period of 8 weeks.
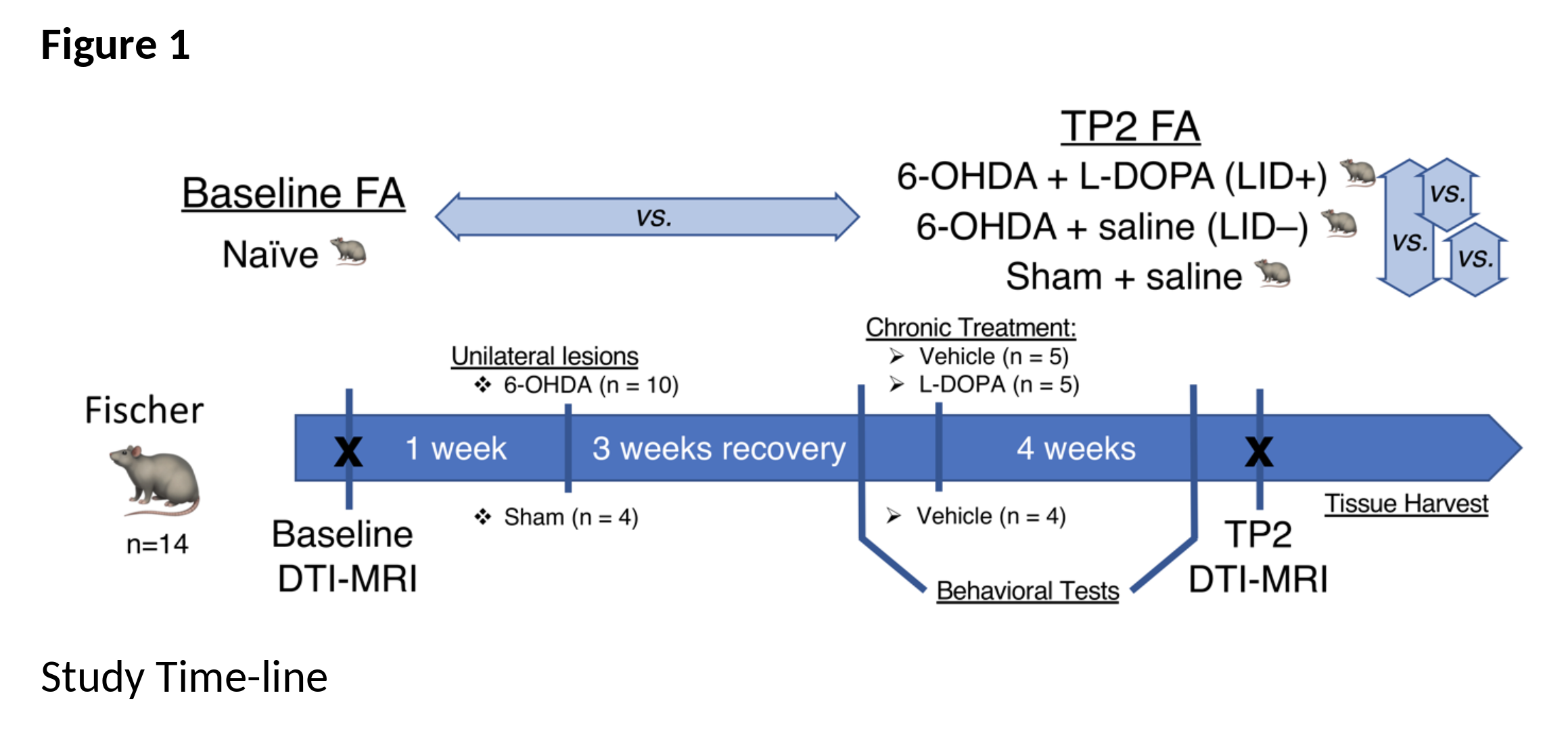
Method: MRI-based DTI was performed at 7T (Bruker). All animals (n=14) [figure1] were scanned at baseline (TP1; naïve); subsequently, subsets of rats were rendered hemi-parkinsonian using unilateral injections of 6-OHDA, or vehicle (sham), into the medial forebrain bundle and substantia nigra. After 6 weeks, 6-OHDA and sham rats received daily injections for 2 weeks of either saline or a LID-inducing L-DOPA regimen. Subsequently, all animals were scanned again OFF and ON L-DOPA. DTI analysis was performed in MRtrix3 [5] and FSL [6]. Two-way mixed ANOVA analysis was used to analyze effects of group, time and their interaction. Post-hoc analyses for time and group were also investigated.
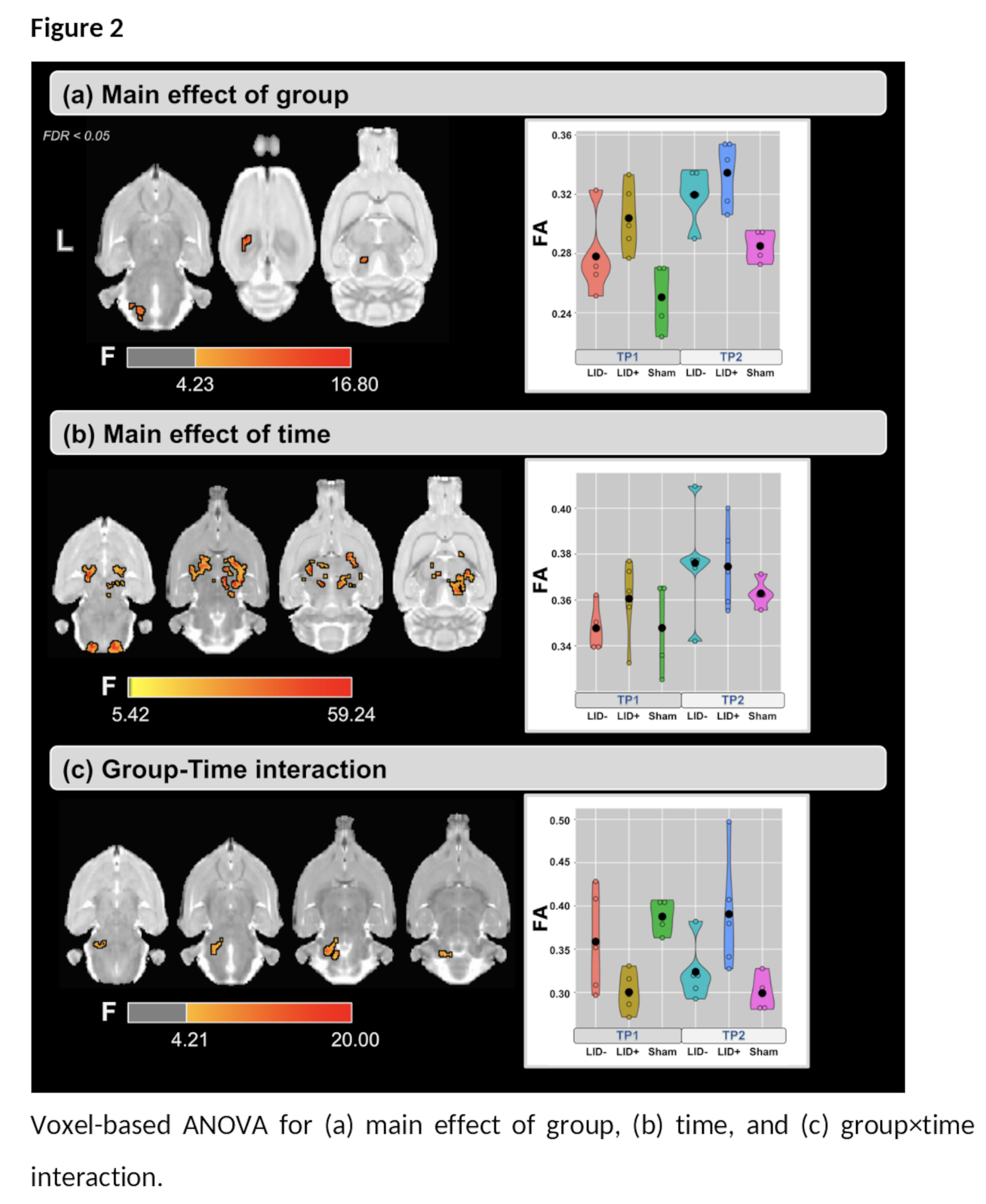
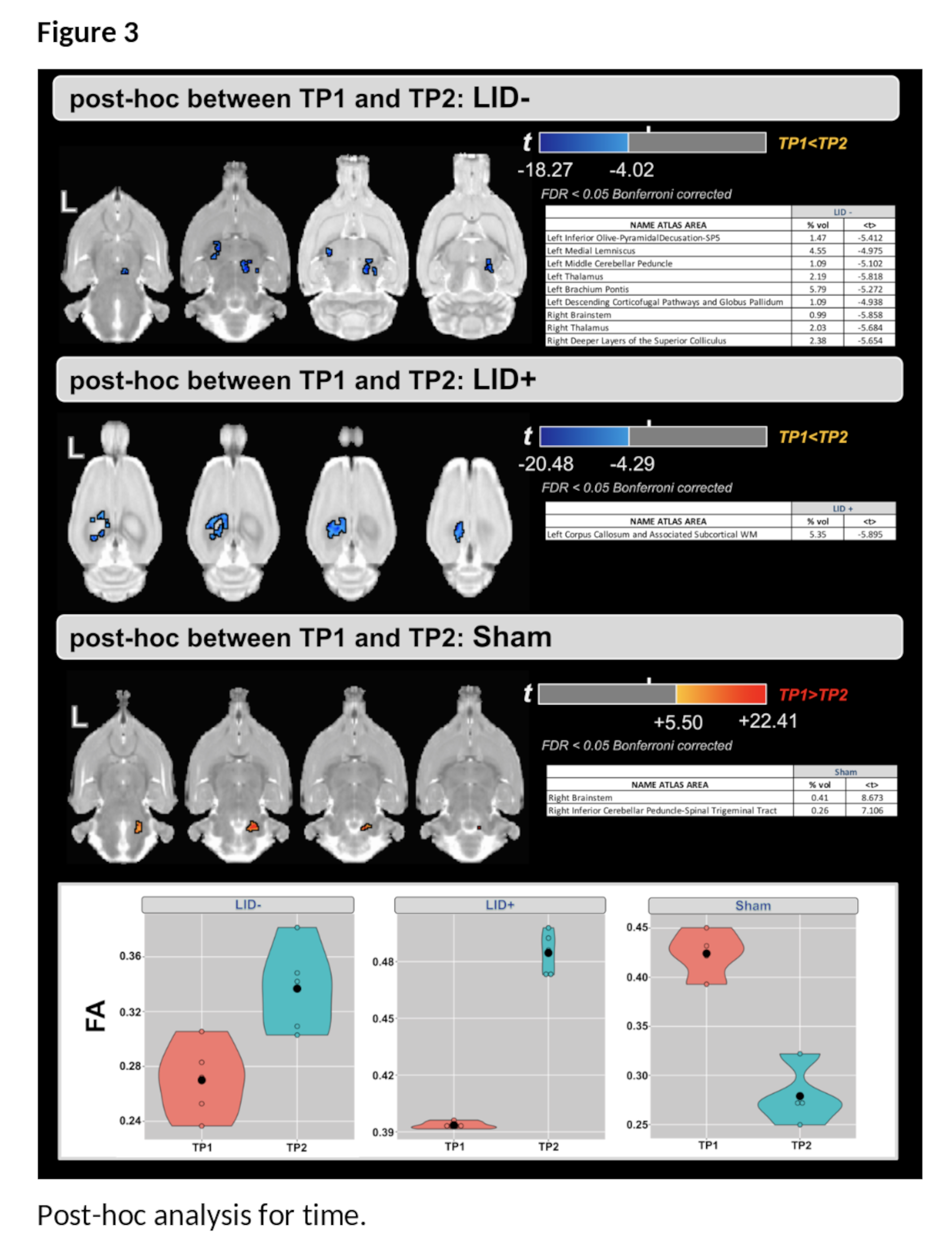
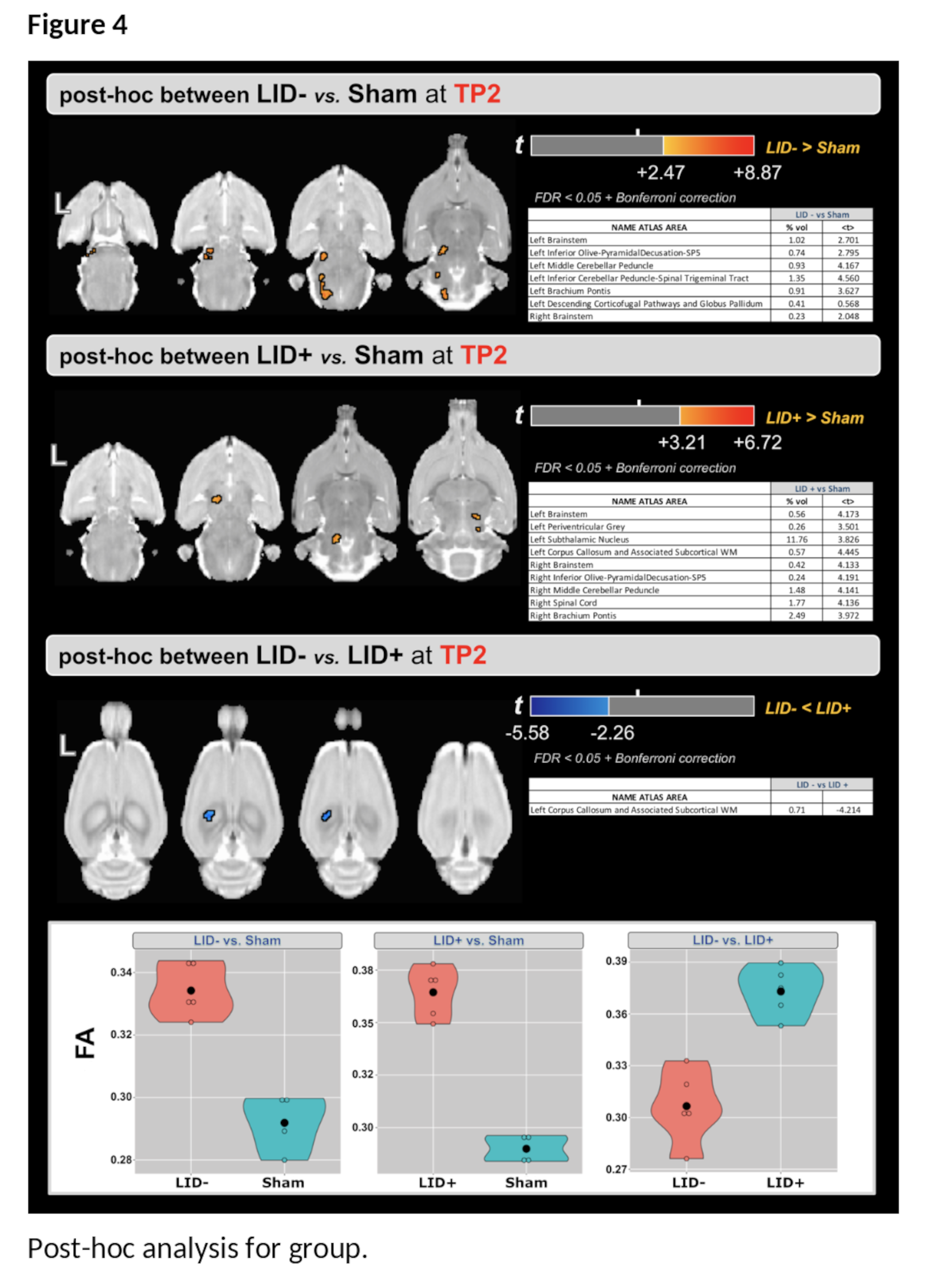
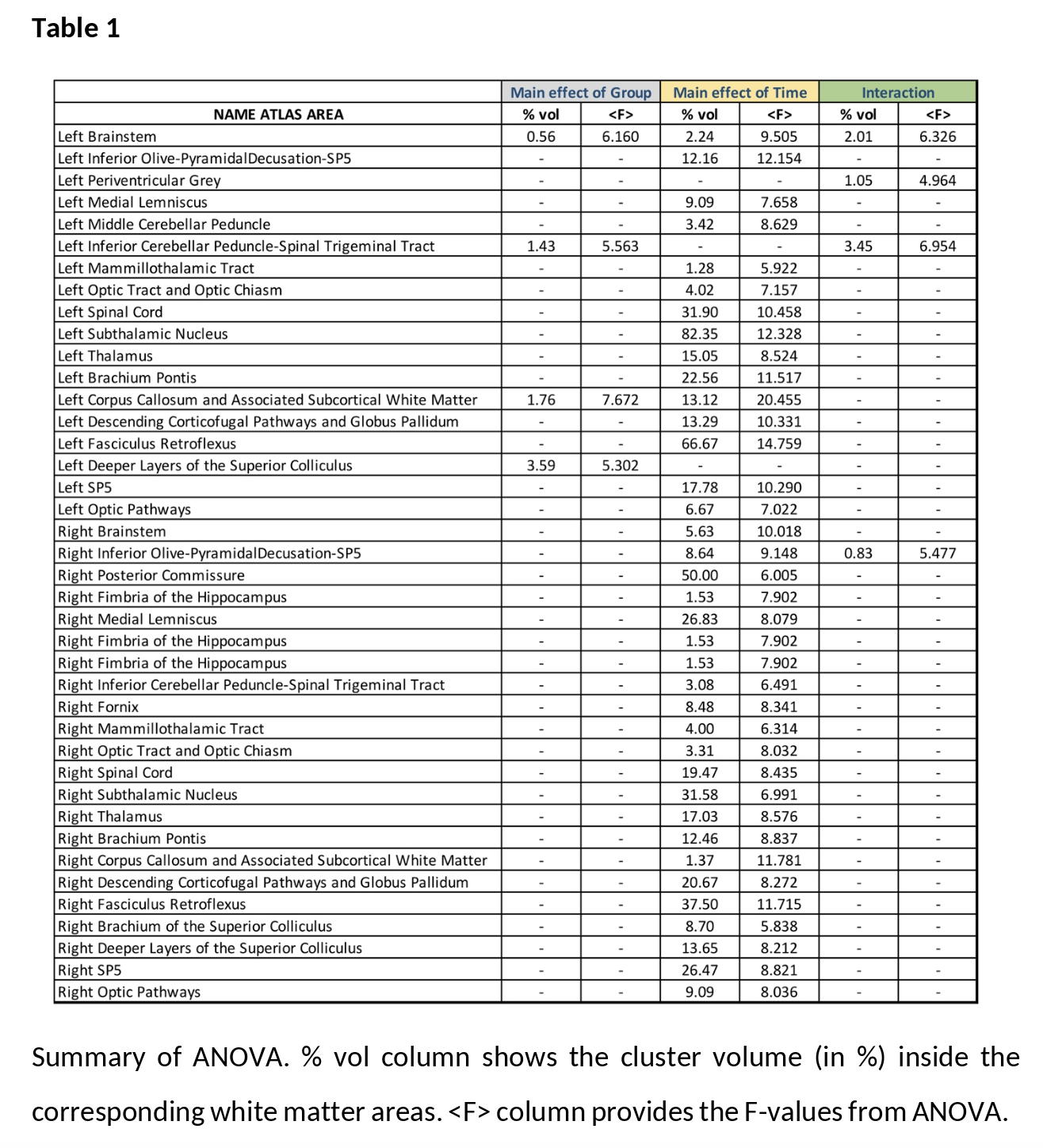
Results: For the main effect of group, higher values of FA were found in the LID+ group compared with sham and LID- groups at both time-points ([figure2-a] and [table1]). For the main effect of time, higher values of FA were found at TP2 in several WM areas [Figure2-b]. Finally, the group×time interaction (Figure2-c) shows predominantly cerebellar changes. Post-hoc analysis for time shows that FA increases in both LID groups [figure3], while LID+ is associated with higher FA than both LID- and sham groups [figure4].
Conclusion: In this preliminary study, we observed microstructural changes in WM associated with 6-OHDA lesions and LID, with comparison to pre-lesion epochs and sham rats. Work is ongoing work to assess behavioral correlates of altered WM microstructure. The findings in this abstract will also be presented at the ISMRM conference in May 2022.
References: [1] Ascherio A, Schwarzschild MA. The epidemiology of Parkinson’s disease: risk factors and prevention. Lancet Neurol. Nov 2016;15(12):1257-1272. doi:10.1016/S1474-4422(16)30230-7
[2] Manson A, Stirpe P, Schrag A. Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J Parkinsons Dis. 2012;2(3):189-98. doi:10.3233/JPD-2012-120103
[3] Ogawa T, Hatano T, Kamagata K, et al. White matter alterations in Parkinson’s disease with levodopa-induced dyskinesia. Parkinsonism Relat Disord. Sep 2021;90:8-14. doi:10.1016/j.parkreldis.2021.07.021
[4] Perlbarg V, Lambert J, Butler B, et al. Alterations of the nigrostriatal pathway in a 6-OHDA rat model of Parkinson’s disease evaluated with multimodal MRI. PLoS One. 2018;13(9):e0202597. doi:10.1371/journal.pone.0202597
[5] J.-D. Tournier, R. E. Smith, D. Raffelt, R. Tabbara, T. Dhollander, M. Pietsch, D. Christiaens, B. Jeurissen, C.-H. Yeh, and A. Connelly. MRtrix3: A fast, flexible and open software framework for medical image processing and visualisation. NeuroImage, 202 (2019), pp. 116–37.
[6] M. Jenkinson, C.F. Beckmann, T.E. Behrens, M.W. Woolrich, S.M. Smith. FSL. NeuroImage, 62:782-90, 2012
To cite this abstract in AMA style:
M. Bergamino, A. Fuentes, D. Marmion, I. Sandoval, C. Bishop, F. Manfredsson, A. Stokes. Assessment of microstructural changes associated with levodopa-induced dyskinesia in hemiparkinsonian rodents [abstract]. Mov Disord. 2022; 37 (suppl 2). https://www.mdsabstracts.org/abstract/assessment-of-microstructural-changes-associated-with-levodopa-induced-dyskinesia-in-hemiparkinsonian-rodents/. Accessed February 9, 2026.« Back to 2022 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/assessment-of-microstructural-changes-associated-with-levodopa-induced-dyskinesia-in-hemiparkinsonian-rodents/