Category: Tremor
Objective: To understand patient characteristics and real-world medication patterns of patients with essential tremor (ET) being initiated on treatment in the US.
Background: ET is a common movement disorder[1] that is estimated to affect over a million people in the US.[2] ET is often undertreated/untreated due to challenges with diagnosis and/or limited treatment options[3]; propranolol is the only medication approved in the US to treat ET.[4, 5] Per guidelines, first-line ET medications have efficacy and/or safety limitations, and several off-label ET medications should be avoided or used with caution.[6]
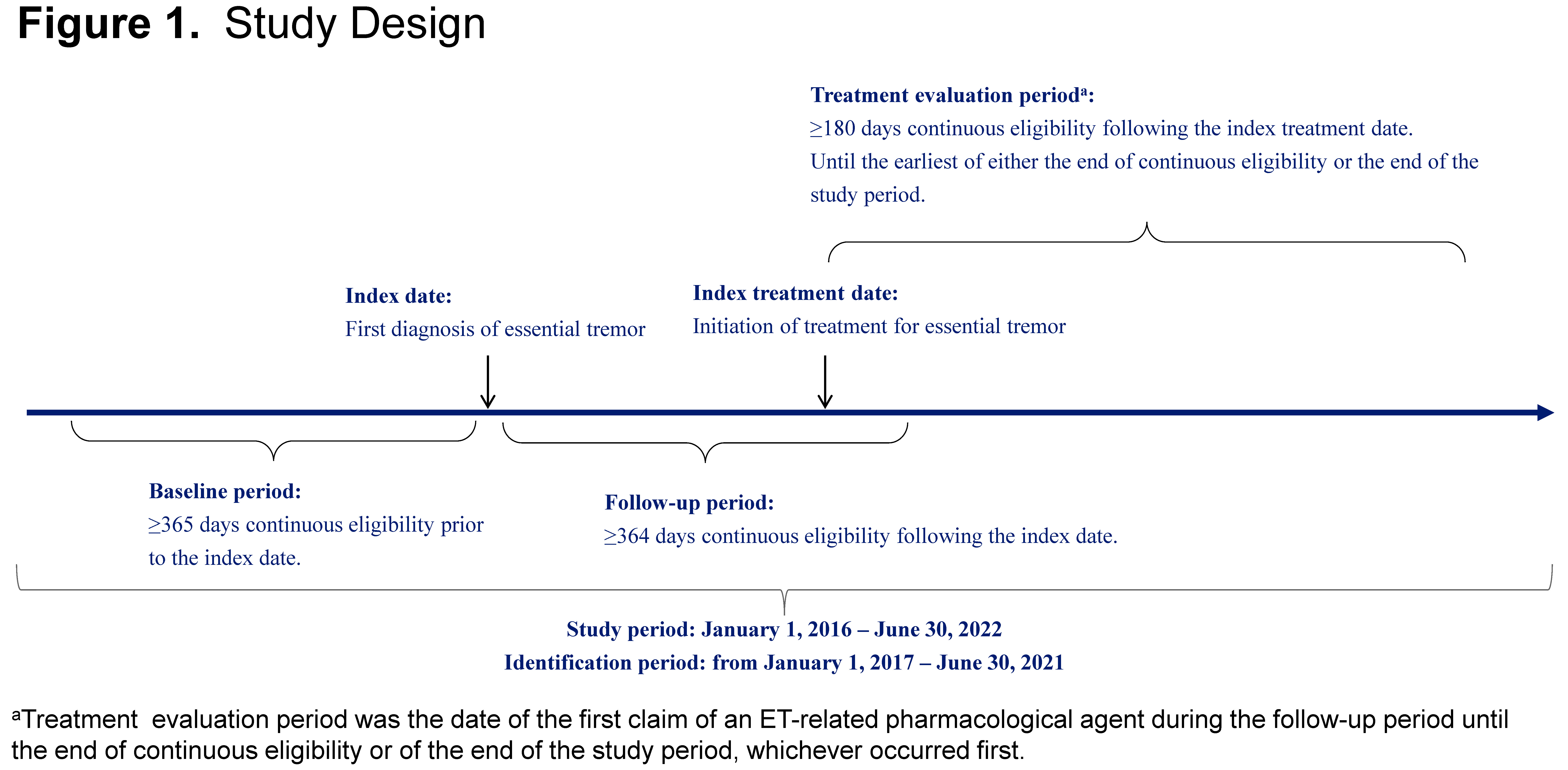
Method: Merative™ MarketScan® administrative claims were analyzed (study period, 1/1/2016-6/30/2022). Eligible patients were ≥18 years, entered the cohort upon receipt of first ET diagnosis (index date), had continuous medical and pharmacy coverage ≥365 days prior (baseline) and ≥364 days after index date (follow-up), and had no pre-existing ET diagnosis in the baseline period [figure1]. Descriptive analyses were performed for patient and clinical characteristics, line of therapy, and switch patterns.
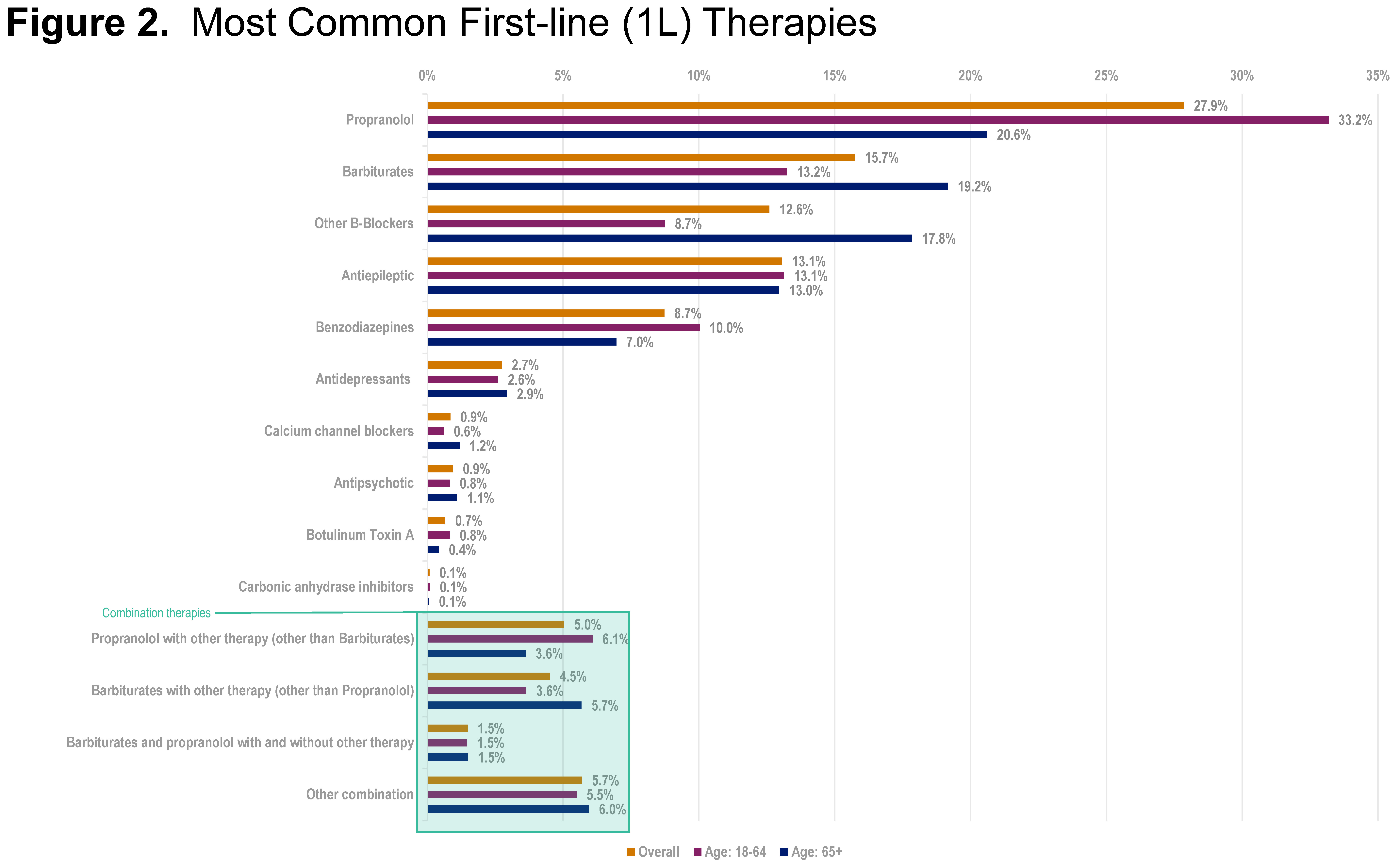
Results: In total, 18,177 patients were included, with 14,425 receiving a medication in the follow-up period (<65 years, n=8334; ≥65 years, n=6091). Depression and anxiety were seen in a higher proportion of patients <65 (23%; 22%) than those ≥65 years (18%; 15%). Among treated patients, median (IQR) treatment evaluation period lasted 841 (560-1233) days, during which 37% of patients received 1 line of therapy, 14% were prescribed 2 lines, and 49% received ≥3 lines of therapy. Overall, propranolol was most frequently used as first-line therapy (1L), with barbiturates and antiepileptics the most frequent off-label medications used [figure2]. Median (IQR) time from diagnosis to 1L initiation was 9 (0-37) days, with median (IQR) duration for 1L being 166 (35-540) days. Over 60% of patients advanced from 1L to 2L, with a median (IQR) 2L duration of 79 (30-240) days.
Conclusion: This study found that most patients with ET may require >1 line of therapy and evolve their medication regimen with a shorter median time spent on 2L compared with 1L. Also, ET treatment included a high use of off-label medications that might have limited efficacy and poor tolerability profiles.[1, 6] These results emphasize the challenges with currently available medication options in ET.
Figure 1
Figure 2
References: 1. Louis ED, McCreary M. Tremor Other Hyperkinet Mov (N Y). 2021;11:28.
2. Louis ED, Ottman R. Tremor Other Hyperkinet Mov (N Y). 2014;4:259.
3. Ferreira JJ, Mestre TA, Lyons KE, et al. Mov Disord. 2019;34(7):950-8.
4. Zesiewicz T, Vega J, Gooch C, et al. Mov Disord Clin Pract. 2022;9(6):728-34.
5. Inderal [package insert]. Philadelphia, PA: Wyeth Pharmaceuticals Inc, a subsidiary of Pfizer Inc; 2017.
6. American Academy of Neurology. Update: Treatment of Essential Tremor. AAN Summary of Evidence-based Guidelines for Clinicians. 2011. Available at: https://www.aan.com/Guidelines/home/GuidelineDetail/492. Accessed March 5, 2024.
To cite this abstract in AMA style:
J. Williams, R. Pahwa, L. Barbato, J. Lin, F. Tefos, A. Sillah, S. Thomas, F. Geng, Y. Pan, J. Friderici, S. Shah. Characteristics and Treatment Patterns of Patients Newly Diagnosed With Essential Tremor: A Retrospective Claims Database Analysis in the US [abstract]. Mov Disord. 2024; 39 (suppl 1). https://www.mdsabstracts.org/abstract/characteristics-and-treatment-patterns-of-patients-newly-diagnosed-with-essential-tremor-a-retrospective-claims-database-analysis-in-the-us/. Accessed July 1, 2025.« Back to 2024 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/characteristics-and-treatment-patterns-of-patients-newly-diagnosed-with-essential-tremor-a-retrospective-claims-database-analysis-in-the-us/