Session Information
Date: Monday, September 23, 2019
Session Title: Rare Genetic and Metabolic Diseases
Session Time: 1:45pm-3:15pm
Location: Les Muses Terrace, Level 3
Objective: Perform a comprehensive clinical characterization and biochemical cerebrospinal fluid (CSF) profile analyses in two Swedish families with hereditary spastic paraparesis 10 (SPG10) caused by two different mutations in the kinesin heavy chain gene (KIF5A).
Background: Hereditary spastic paraparesis (HSP) comprises a large group of chronic progressive neurodegenerative diseases with varying pattern of inheritance and disease severity, but all sharing an affection of corticospinal tracts. Heterozygous mutations in KIF5A are associated with autosomal dominant HSP 10 (SPG10) [1]. KIF5A encodes one of two heavy chain subunits that are necessary for the formation of a tetrameric kinesin-1 protein, thought to be crucial for molecular axonal transport by binding to microtubule as shown in vitro [2]. Mutations in KIF5A are thus believed causative of an axonopathy involving the central and peripheral nervous system [1, 3].
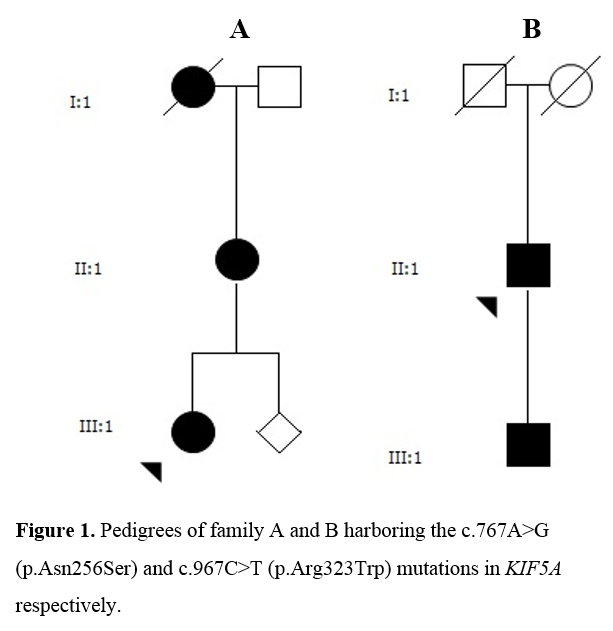
Method: Structured clinical assessment, genetic studies, neuroradiological and electrophysiological evaluations were performed in four patients from two families with SPG10 (Figure 1). CSF analysis was conducted in three patients with regard to levels of neurodegenerative markers and monoamine metabolism. [figure1]
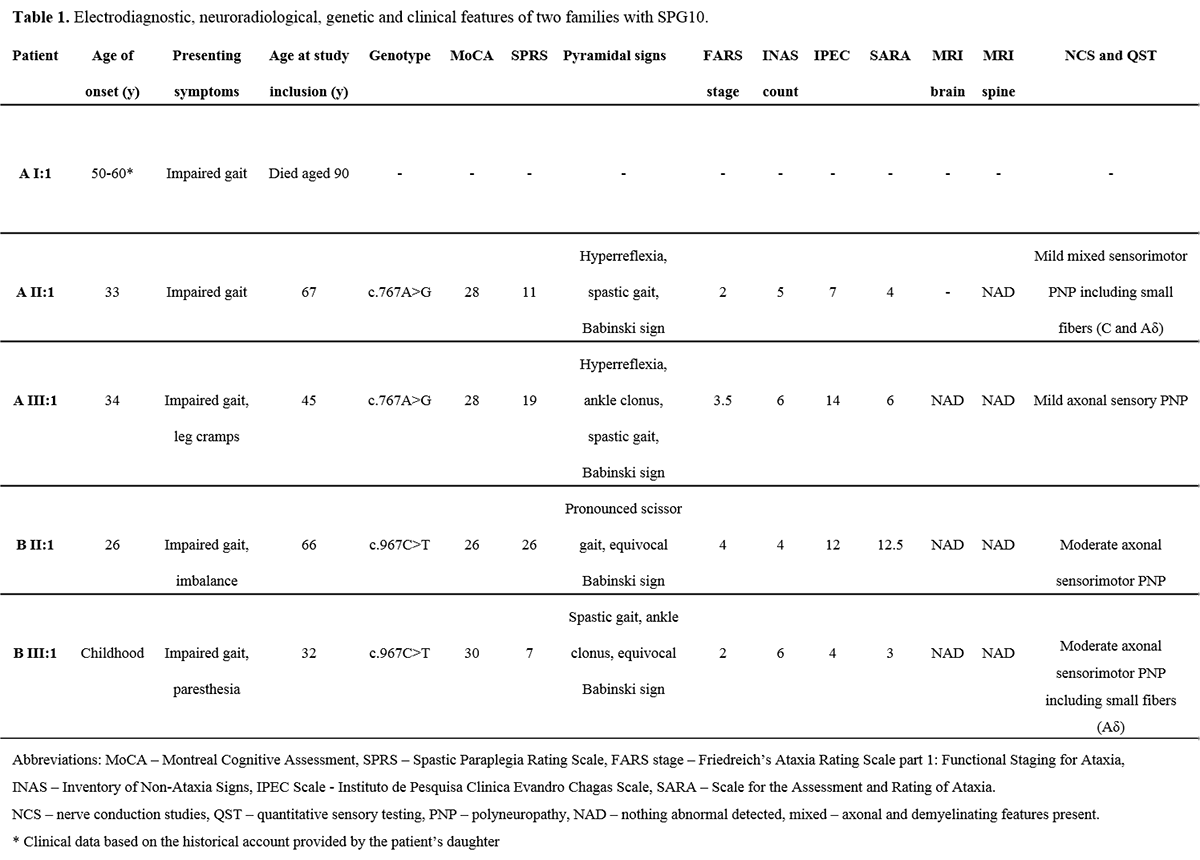
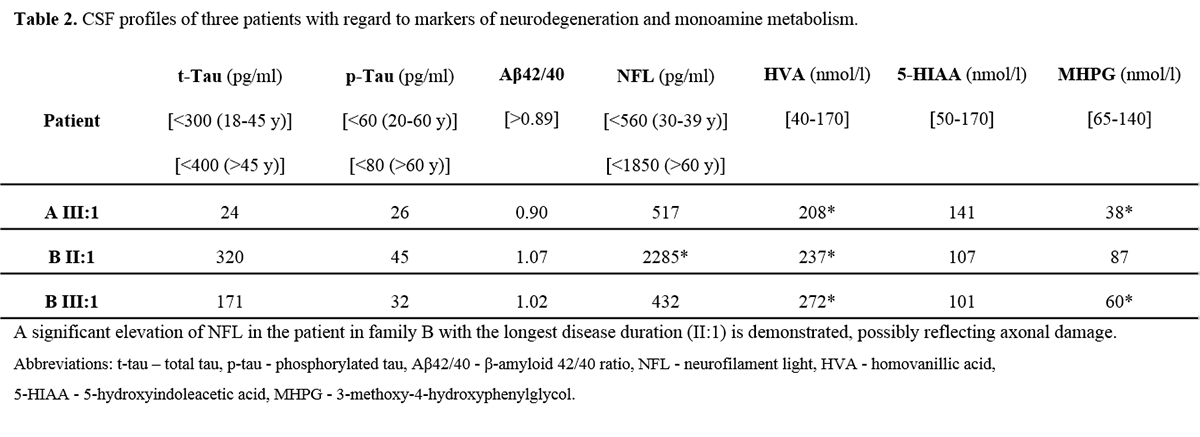
Results: All patients exhibited a complex form of HSP with mild to moderate concurrent axonal polyneuropathy. The heterozygous KIF5A mutations c.767A>G and c.967C>T were found. Clinical data shown in Table 1. [table1] CSF analysis demonstrated a mild elevation of neurofilament light (NFL) chain in the patient with longest disease duration. All patients exhibited increased levels of the dopamine metabolite, homovanillic acid, whereas decreased levels of the noradrenergic metabolite, 3-methoxy-4-hydroxyphenylglycol, were found in two of three patients. CSF data shown in Table 2. [table2]
Conclusion: This is the first time CSF abnormalities are reported in SPG10. Biochemically, NFL elevation is not a mandatory CSF finding in SPG10 but may appear after longstanding disease. Impaired transportation of synaptic proteins may be a possible explanation for the increased dopaminergic turn-over and noradrenergic deficiency identified. The reasons for these selective abnormalities, unrelated to obvious clinical features, remain to be explained. Our findings need further confirmation in larger cohorts of patients harboring KIF5A mutations.
References: [1] Reid E, Kloos M, Ashley-Koch A, et al. A kinesin heavy chain (KIF5A) mutation in hereditary spastic paraplegia (SPG10). Am J Hum Genet 2002;71:1189-1194. [2] Ebbing B, Mann K, Starosta A, et al. Effect of spastic paraplegia mutations in KIF5A kinesin on transport activity. Hum Mol Genet 2008;17:1245-1252. [3] Rinaldi F, Bassi MT, Todeschini A, et al. A novel mutation in motor domain of KIF5A associated with an HSP/axonal neuropathy phenotype. J Clin Neuromuscul Dis 2015;16:153-158.
To cite this abstract in AMA style:
M. Andréasson, K. Lagerstedt-Robinson, K. Samuelsson, G. Solders, K. Blennow, M. Paucar, P. Svenningsson. Characterization of cerebrospinal fluid profile in hereditary spastic paraparesis 10 [abstract]. Mov Disord. 2019; 34 (suppl 2). https://www.mdsabstracts.org/abstract/characterization-of-cerebrospinal-fluid-profile-in-hereditary-spastic-paraparesis-10/. Accessed July 5, 2025.« Back to 2019 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/characterization-of-cerebrospinal-fluid-profile-in-hereditary-spastic-paraparesis-10/