Session Information
Date: Sunday, October 7, 2018
Session Title: Restless Legs Syndrome and Other Sleep Disorders
Session Time: 1:45pm-3:15pm
Location: Hall 3FG
Objective: To determine the efficacy and safety of clonazepam for the treatment of rapid eye movement (REM) sleep behavior disorder (RBD) in patients with Parkinsonism.
Background: Clonazepam is treatment of choice for RBD which is frequent non-motor symptom in patients with Parkinsonism. However, there have been no randomized controlled trials that evaluated the efficacy of clonazepam compared with placebo.
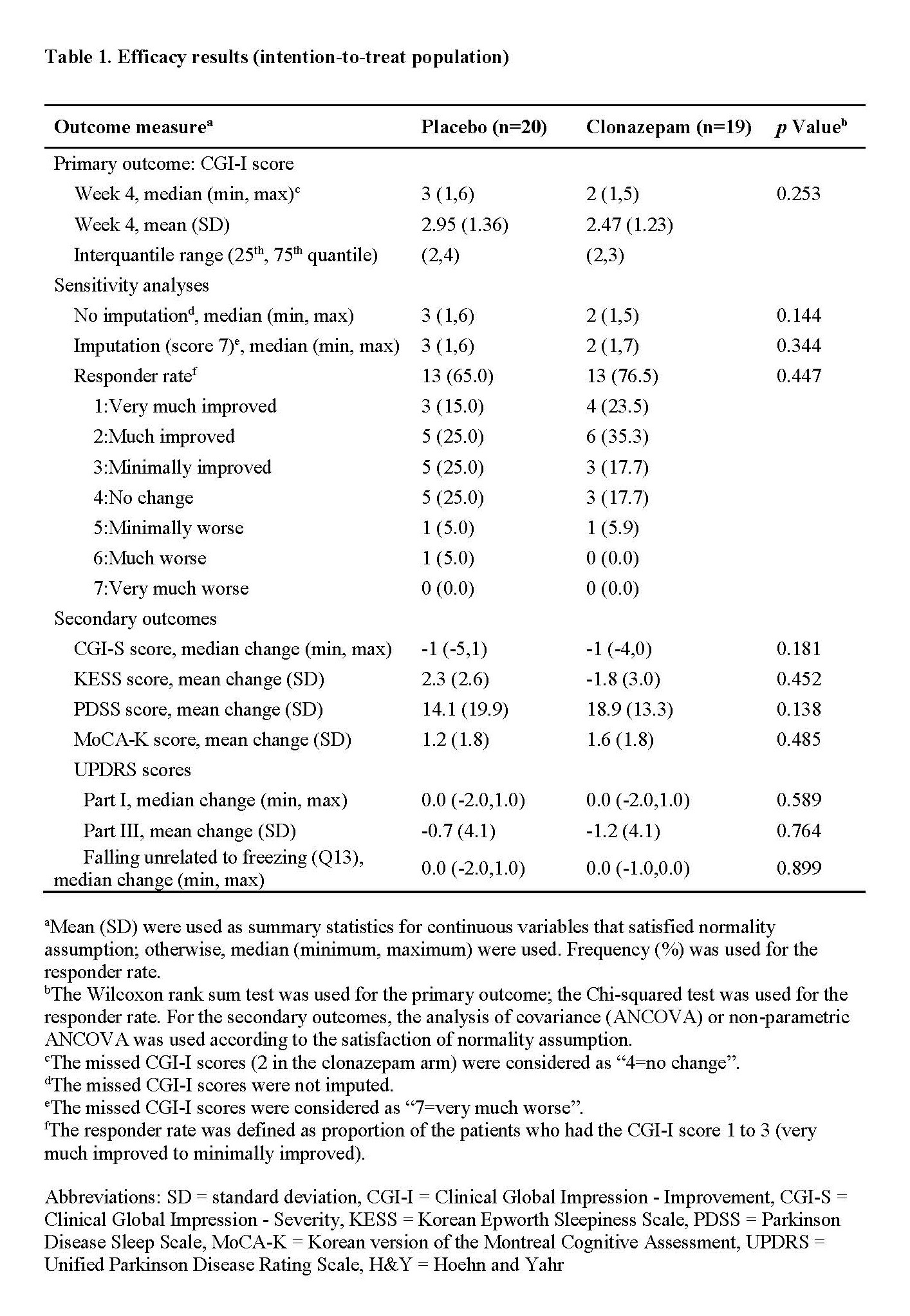
Methods: We conducted a 4-week randomized, double-blind, placebo-controlled trial of clonazepam (0.5mg a day) vs placebo for RBD symptoms in patients with Parkinsonism. Patients aged 30 years or more with a caregiver, who could observe RBD symptoms, were recruited between April 2015 and February 2016. The primary outcome was the Clinical Global Impression-Improvement (CGI-I) score at week 4 comparing clonazepam and placebo group. The secondary outcomes were the changes from baseline to primary endpoint of the CGI-Severity (CGI-S) score, Korean Epworth Sleepiness Scale (KESS), Parkinson Disease Sleep Scale (PDSS), Montreal Cognitive Assessment (MoCA), Unified Parkinson Disease Rating Scale (UPDRS), and Hoehn and Yahr scale. For safety analysis, frequency of adverse events was compared between two groups. Statistical analyses were performed in intention-to-treatment population.
Results: Total 40 patients were enrolled, and 20 were assigned to receive clonazepam and 20 to receive placebo. All patients were diagnosed as PD. Three subjects in clonazepam group withdrew after randomization; 1 withdrew informed consent before receiving allocated intervention and was excluded from intention-to-treat population, 2 discontinued during the follow-up due to following reasons: 1 due to daytime sleepiness and 1 due to dyskinesia aggravation. At week 4, there was no significant difference on the CGI-I score between clonazepam (median score [minimum, maximum] = 2 [1,5]) and placebo group (3 [1,6], p = 0.254; Table 1). All secondary outcomes and frequency of adverse events were not significantly different between clonazepam and placebo group. No serious adverse events were observed and the most frequent adverse event was daytime somnolence by clonazepam treatment.
Conclusions: For the treatment of RBD in patients with Parkinsonism, clonazepam was not effective than placebo. This study emphasized the importance of large-scale randomized trials to prove the benefit of clonazepam, which is believed as the first-line treatment in routine clinical practice. A better outcome measurement for severity of RBD and assistance of polysomnography are needed in future clinical trials.
References: 1. Aurora RN, Zak RS, Maganti RK, et al. Best practice guide for the treatment of REM sleep behavior disorder (RBD). Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine 2010;6:85-95. 2. Schenck CH, Montplaisir JY, Frauscher B, et al. Rapid eye movement sleep behavior disorder: devising controlled active treatment studies for symptomatic and neuroprotective therapy–a consensus statement from the International Rapid Eye Movement Sleep Behavior Disorder Study Group. Sleep medicine 2013;14:795-806.
To cite this abstract in AMA style:
C. Shin, H. Park, W. Lee, B. Jeon, H. Kim. Clonazepam for REM sleep behavior disorder in Parkinsonism: A randomized, placebo-controlled trial [abstract]. Mov Disord. 2018; 33 (suppl 2). https://www.mdsabstracts.org/abstract/clonazepam-for-rem-sleep-behavior-disorder-in-parkinsonism-a-randomized-placebo-controlled-trial/. Accessed January 16, 2026.« Back to 2018 International Congress
MDS Abstracts - https://www.mdsabstracts.org/abstract/clonazepam-for-rem-sleep-behavior-disorder-in-parkinsonism-a-randomized-placebo-controlled-trial/